A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|32 VideosELECTROCHEMISTRY
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 1|68 VideosD - AND F-BLOCK ELEMENTS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|27 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|14 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ELECTROCHEMISTRY-Exercise 2
- For pure water degree of dissociation of water is 1.9xx10^(-9) we...
Text Solution
|
- The efficiency of a fuel cell is 80 % and the standard heat of reatc...
Text Solution
|
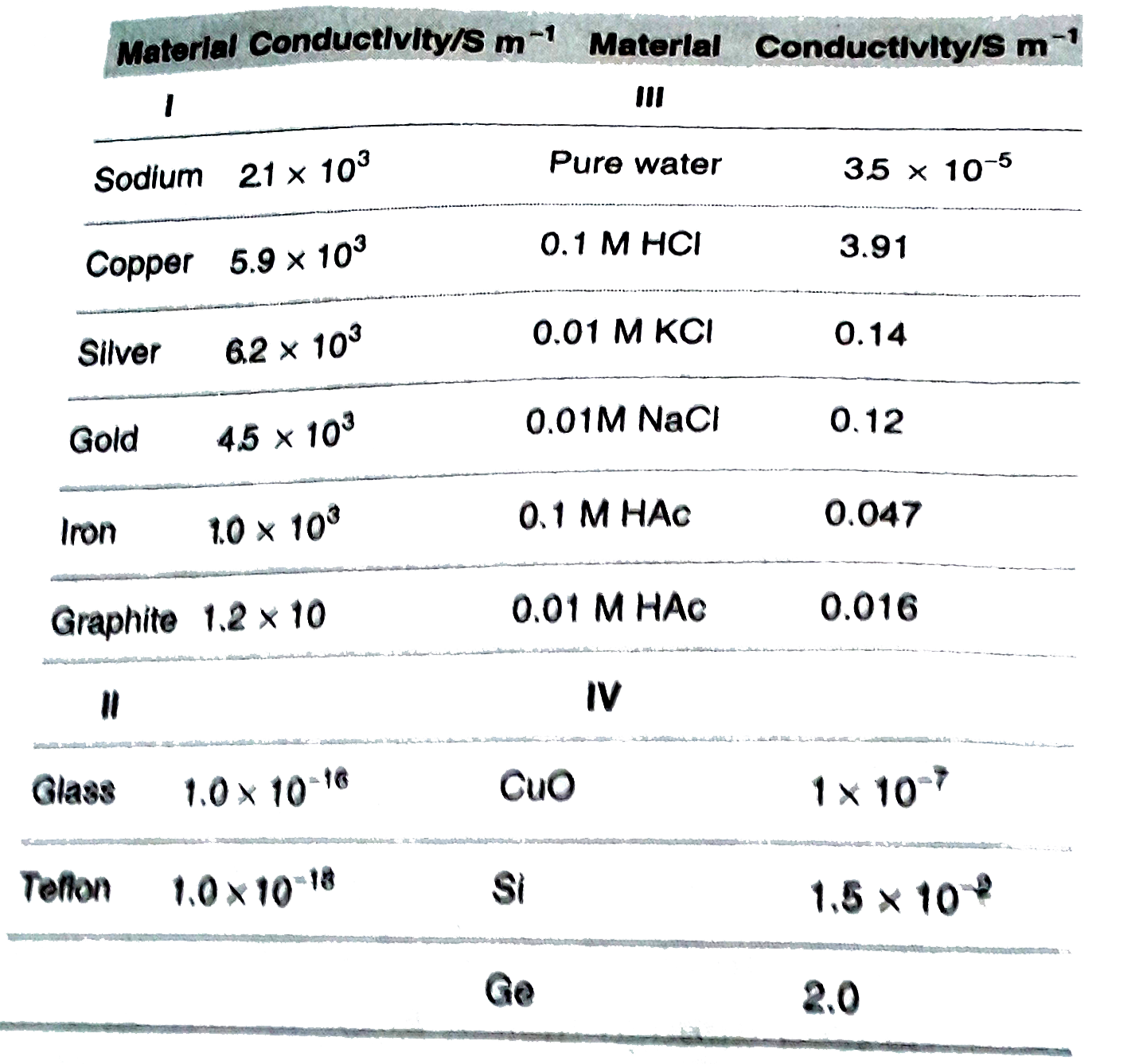
- Consider the following table What is I,II,III,IV in the above tab...
Text Solution
|
- Match the items of column I and column II and choose the correct op...
Text Solution
|
- The logarithm of the equilibriium constant of the cell reaction cor...
Text Solution
|
- At 25^(@)C temperature the cell potential of a given electrochemica...
Text Solution
|
- For the following cell reaction Ag|Ag^(+)|AgCI|CI^(-)|CI(2) Pt ...
Text Solution
|
- Two concentration cells of Ag with Ag electrode in AgNO(3) in fi...
Text Solution
|
- The zinc / siver oxide cell is used in electric watches Zn rarr Zn^...
Text Solution
|
- E(1),E(2) and E(3) are the emf of the following three galvancic cell...
Text Solution
|
- Small quantities of compounds TX, TY and TZ are put into separate test...
Text Solution
|
- E(Fe^(3+)//Fe)^(@)=-0.036V,E(Fe^(2+)//Fe)^(@)=-0.0439V. The value of s...
Text Solution
|
- A device that convers energy of combustion of fueles like hydrogen and...
Text Solution
|
- An alloy of Pb Ag weighing 1.08 g was dissolved in dilute HNO(3) and ...
Text Solution
|
- The standard oxidation potentials of Zn,Cu Ag and Ni electrodes are +...
Text Solution
|
- The standard emf of a galvanic cell involving cell reaction with...
Text Solution
|
- The standar reduction potentials of Zn^(2+)|Zn,Cu^(2+)||Cu^(2+)|Cu| Zn...
Text Solution
|
- The Edision storage cell is represented as Fe(s)|FeO(s)|KOH(aq)|Ni(2...
Text Solution
|
- The standard reduction potentials at 298 K for the following half ...
Text Solution
|
- Which of the following reaction cannot be a base for electrochemi...
Text Solution
|