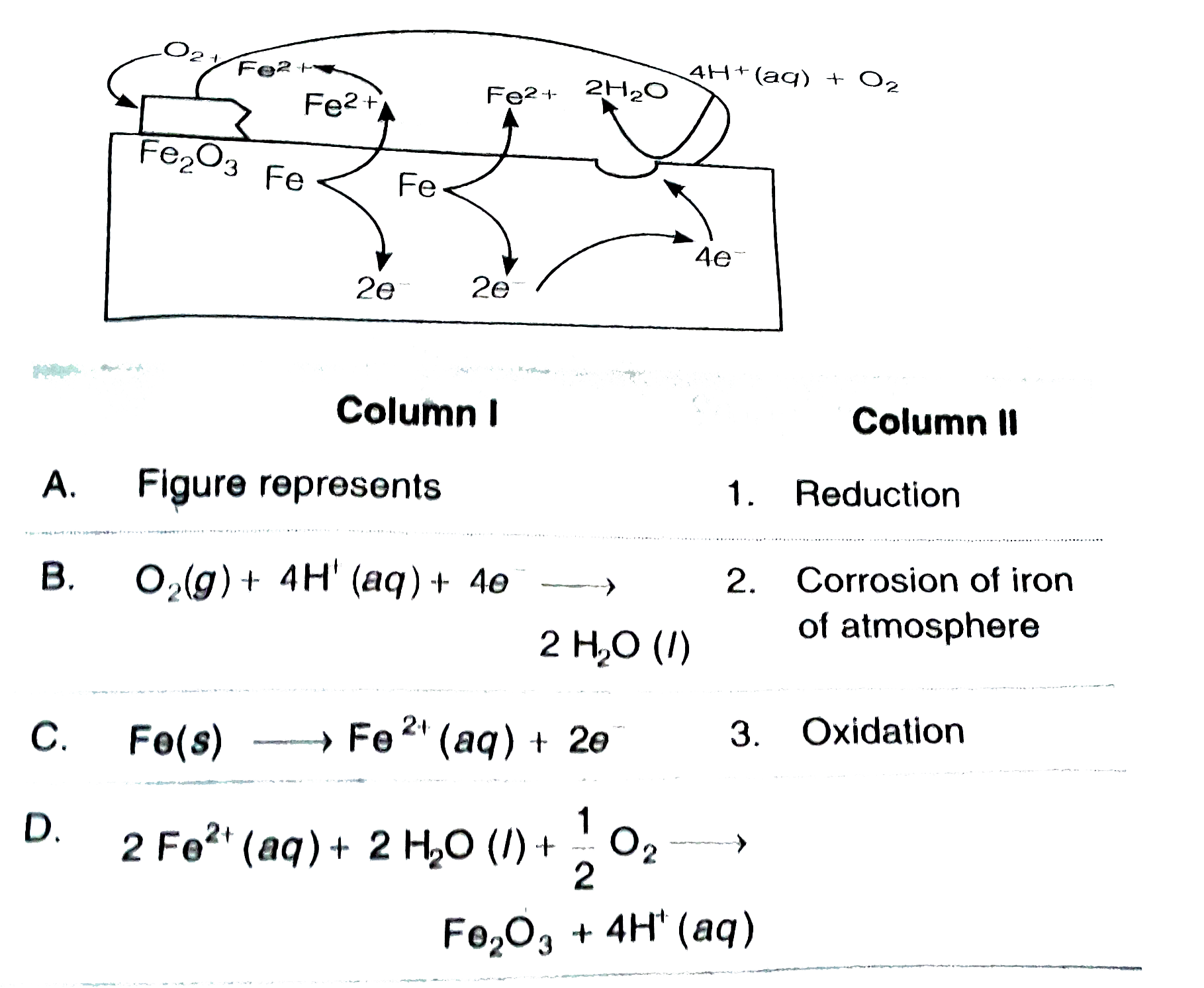
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ELECTROCHEMISTRY
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|32 VideosELECTROCHEMISTRY
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 1|68 VideosD - AND F-BLOCK ELEMENTS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|27 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|14 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ELECTROCHEMISTRY-Exercise 2
- For hydrogen oxygen fuel cell at atm and 298 K H(2)g+1/2O(2)(g)rarrH...
Text Solution
|
- which of the following statement is true for the electrochemical ...
Text Solution
|
- Standard free energies fo formation (in kJ/ mol) at 298 K are -237.2 -...
Text Solution
|
- Which of the followng reaction is used to make a fuel cell
Text Solution
|
- The cell reaction of the galvanic cell Cu(s)|Cu^(2+)(aq)||Hg^(2+)(aq...
Text Solution
|
- Matach column I with column II related to the figure given below and...
Text Solution
|
- Which of the following reaction cannot be a base for electrochemical...
Text Solution
|
- The electrochemical cell stops working after sometimes because
Text Solution
|
- In a hydrogen oxygen fuel cell combustion of hydrogen occurs to
Text Solution
|
- In a galvanic cell the eletrons flow from
Text Solution
|
- In the electrolytic cell flow of electrons is form
Text Solution
|
- What is the anode and cathode in the given in the below
Text Solution
|
- What is the product produced by this cell
Text Solution
|
- Mark the incorrect statement (s) for fuel cell
Text Solution
|
- Which of the following statement (s) is / are true for secondary ce...
Text Solution
|
- Condisder the following reactions at 1100^(@)C (i)2C+O(2) rarr 2C...
Text Solution
|
- E(cell)^(@) for some half cell reaction are given below on the basis o...
Text Solution
|
- The standard electrode potentials for the ractions Ag^(+)(aq)+e^(-) ...
Text Solution
|
- The equation representing the process by which standard reductin po...
Text Solution
|
- The emf of the cell Ni|Ni^(2)(1.0 M)||Ag^(+)(1.0 M)|Ag E^(@) for N...
Text Solution
|