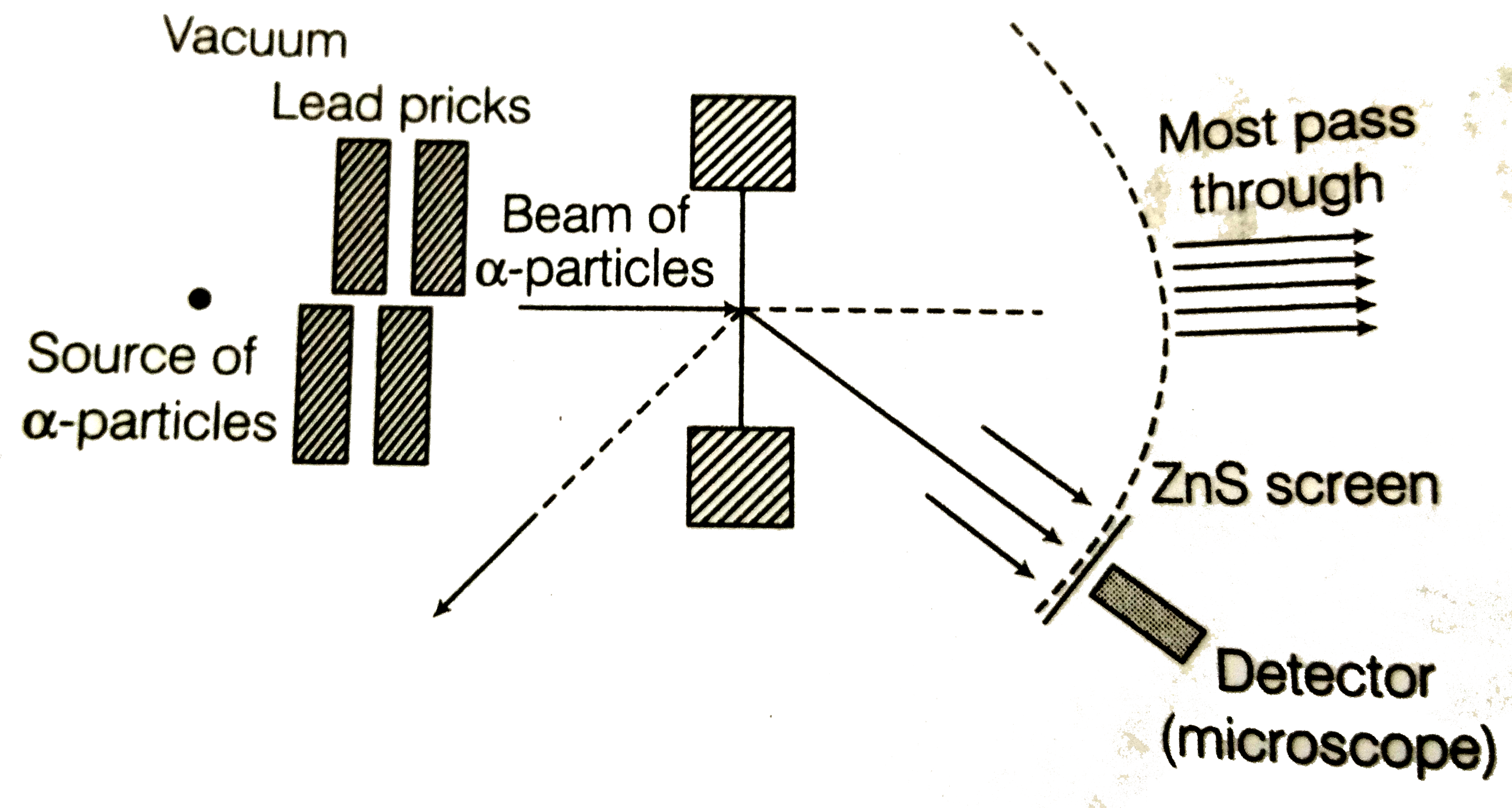
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 2 (MISCELLANEOUS PROBLEMS)|26 VideosATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|30 VideosATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|30 VideosCIRCULAR MOTION
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|21 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ATOMS, MOLECULES AND NUCLEI -Exercise 1 (TOPICAL PROBLEMS)
- In Geiger-Marsden experiment, it can be fairly assumed that the gold n...
Text Solution
|
- In Geiger-Mersden experiment, collimation of alpha-particles into a na...
Text Solution
|
- In Geiger-Mersden experiment, detection of alpha-particles scattered a...
Text Solution
|
- The ionization enegry of the electron in the hydrogen atom in its grou...
Text Solution
|
- If v1 is the frequency of the series limit of lyman seies, v2 is the f...
Text Solution
|
- An electron jumps from the first excited state to the ground stage of ...
Text Solution
|
- In hydrogen atom, an electron jumps from bigger orbit to smaller orbit...
Text Solution
|
- A hydrogen atom ia in excited state of principal quantum number n . I...
Text Solution
|
- In Rutherford scattering experiment, what will b ethe correct angle fo...
Text Solution
|
- An electron is moving in an orbit of a hydrogen atom from which there ...
Text Solution
|
- If lamda(1) and lamda(2) are the wavelengths of the first members of t...
Text Solution
|
- If the electron in the hydrogen atom jumps from third orbit to second ...
Text Solution
|
- Balmer series of hydrogen atom lies in
Text Solution
|
- Consider 3rd orbit of He^(+) (Helium) using nonrelativistic approach t...
Text Solution
|
- What is the wavelength of ligth for the least energetic photon emitted...
Text Solution
|
- The total energy of an electron in 4th orbit of hydrogen atom is
Text Solution
|
- In Rutherford's alpha particle experiment with thin gold foil, 8100 sc...
Text Solution
|
- Consider the spectral line resulting from the transition from n=2 to n...
Text Solution
|
- If and alpha-paricle of mass m, charged q and velocity v is incident o...
Text Solution
|
- The energy of an electron in excited hydrogen atom is -3.4 eV . Then, ...
Text Solution
|