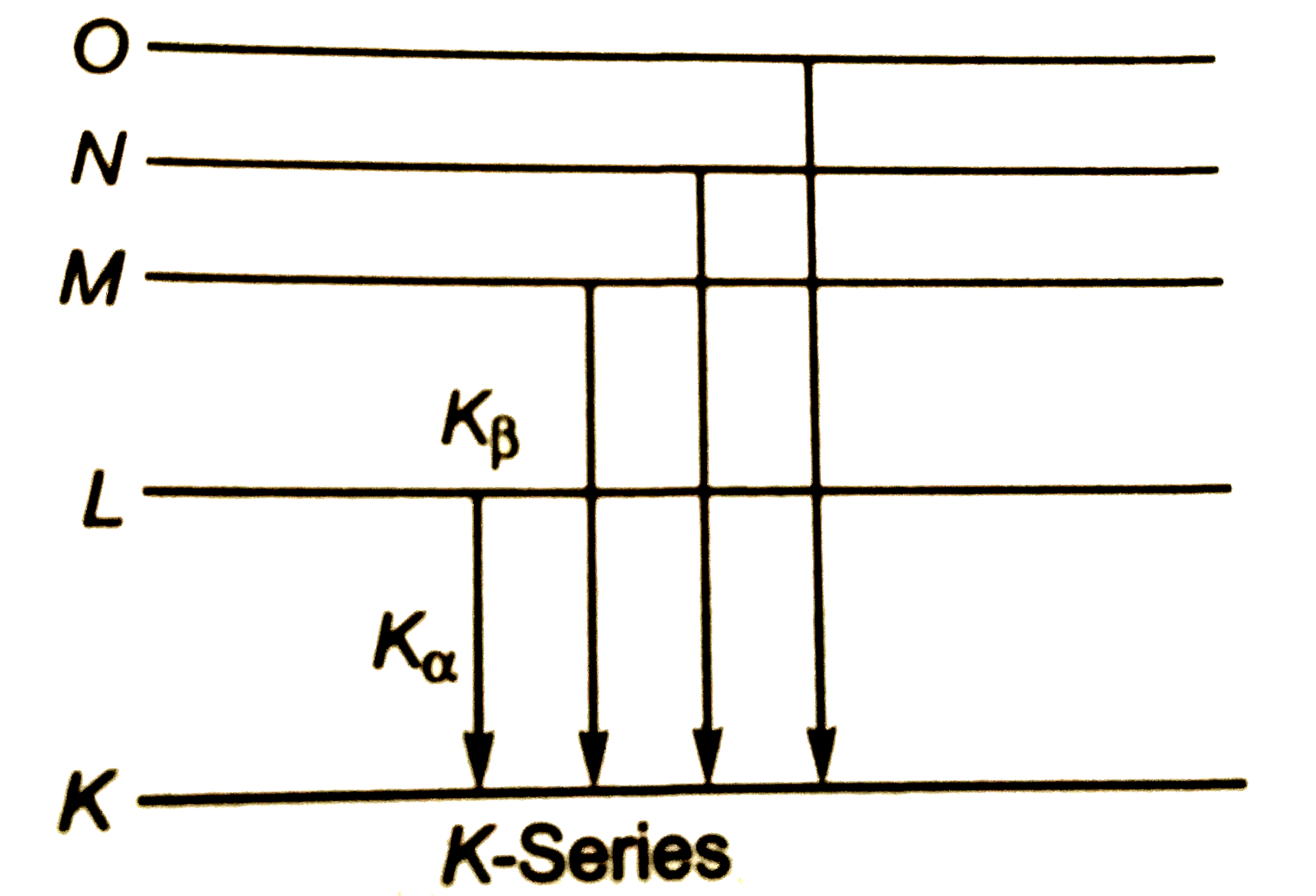
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 2 (MISCELLANEOUS PROBLEMS)|26 VideosATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|30 VideosATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|30 VideosCIRCULAR MOTION
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|21 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ATOMS, MOLECULES AND NUCLEI -Exercise 1 (TOPICAL PROBLEMS)
- When two different materials A and B having atomic number Z(1) and Z(2...
Text Solution
|
- Hard X -rays for the study of fractures in bones should have a minimum...
Text Solution
|
- A beam of 350 keV electrons a molybdenum target, generating the X-rays...
Text Solution
|
- An X-rays of wavelength 0.140 nm are scattered from a block of carbon....
Text Solution
|
- X-ray of wavelength lamda=2 Å is emitted from the metal target. The po...
Text Solution
|
- If a source of power 4 kW produces 10^(20) photons//second, the radiat...
Text Solution
|
- A nucleus splits into two nuclear parts having radii in the ratio 1:2 ...
Text Solution
|
- The mass defect in a particular nuclear reaction is 0.3 grams. The amo...
Text Solution
|
- Number of neutrons in C^(12) and C^(14) are
Text Solution
|
- The density of a nucleus of mass number A is proportional to
Text Solution
|
- The stable nucleus that has a radius 1//3 that of Os^(189) is-
Text Solution
|
- Let binding energy per nucleon of nucleus is denoted by E(bn) and radi...
Text Solution
|
- The nuclear force
Text Solution
|
- If M(A,Z), M(p)and M(n) denote the masses of the nucleus .(Z)X^(A), pr...
Text Solution
|
- What is the Q-value of the reaction? ""^(1)H+""^(7)Lito ""^(4)He+""^...
Text Solution
|
- Binding energy per nucleon relation with mass number
Text Solution
|
- The curve of blinding energy per nucleon as a function of atomic mass ...
Text Solution
|
- Atomic mass of ""6C^(13) is 1300335 amu and its mass number is 13.0 If...
Text Solution
|
- Let N(beta) be the number of beta particles emitted by 1 gram of Na^(2...
Text Solution
|
- A radioactive decay can form an isotope of the original nucleus with t...
Text Solution
|