A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET Corner|30 VideosATOMS, MOLECULES AND NUCLEI
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise Exercise 1 (TOPICAL PROBLEMS)|126 VideosCIRCULAR MOTION
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS|Exercise MHT CET CORNER|21 Videos
Similar Questions
Explore conceptually related problems
MHTCET PREVIOUS YEAR PAPERS AND PRACTICE PAPERS-ATOMS, MOLECULES AND NUCLEI -Exercise 2 (MISCELLANEOUS PROBLEMS)
- In a hypothetical Bohr hydrogen atom, the mass of the electron is doub...
Text Solution
|
- An electron jumps from the 4th orbit to the 2nd orbit of hydrogen atom...
Text Solution
|
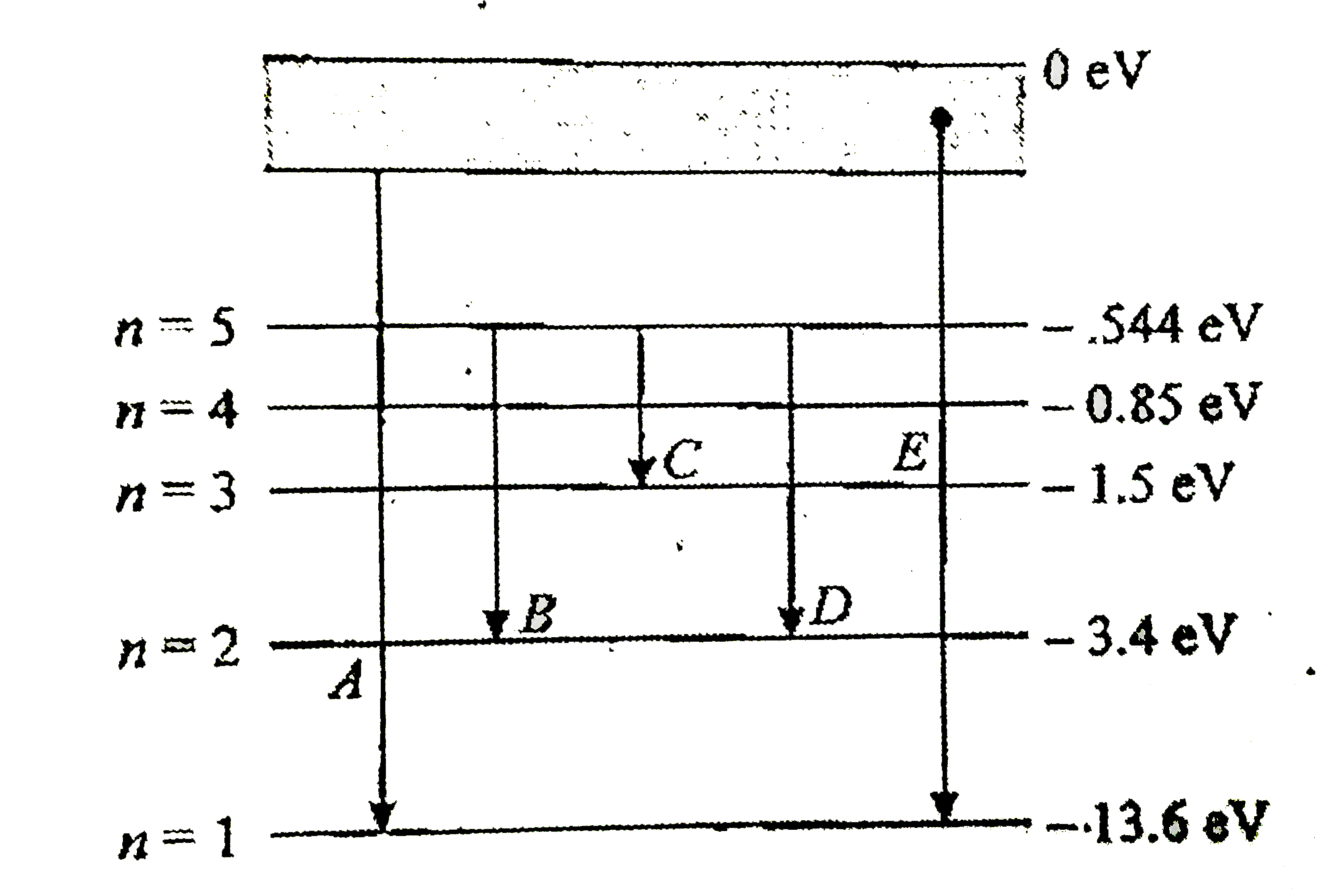
- In the following figure the energy levels of hydroge atom have been sh...
Text Solution
|
- An hydrogen atom moves with a velocity u and makes a head on inelastic...
Text Solution
|
- A hydrogen like ion having wavelength difference between first Balmer ...
Text Solution
|
- In the Bohr's model of hydrogen-like atom the force between the nucleu...
Text Solution
|
- In rutherford's experiment, the mumber of alpha-particles scattered th...
Text Solution
|
- A small particle of mass m move in such a way the potential energy (U ...
Text Solution
|
- In the Bohr model an electron moves in a circular orbit around the pro...
Text Solution
|
- In a hydrogen like atom electron make transition from an energy level ...
Text Solution
|
- If the atom""100Fm^(257) follows the Bohr model the radius of ""100Fm^...
Text Solution
|
- Given a sample of radius -226 having half-life of 4 days. Find, the pr...
Text Solution
|
- Hydrogen atom is exited from ground state to another state with princi...
Text Solution
|
- A diatomic molecule is made of two masses m(1) and m(2) which are sepa...
Text Solution
|
- Suppose an electron is attracted toward the origin by a force(k)/(r ) ...
Text Solution
|
- The largest wavelength in the ultraviolet region of the hydrogen spect...
Text Solution
|
- The filament current in the electron gun of a coolidge tube is increas...
Text Solution
|
- The binding energy of the innermost electron in tungsten is 40 keV. To...
Text Solution
|
- Hydrogen (""(1)H^(1)), Deuterum (""(1)H^(2)), singly ionised Hellium ...
Text Solution
|
- Electrons with energy 80 keV are incident on the tungsten target of an...
Text Solution
|