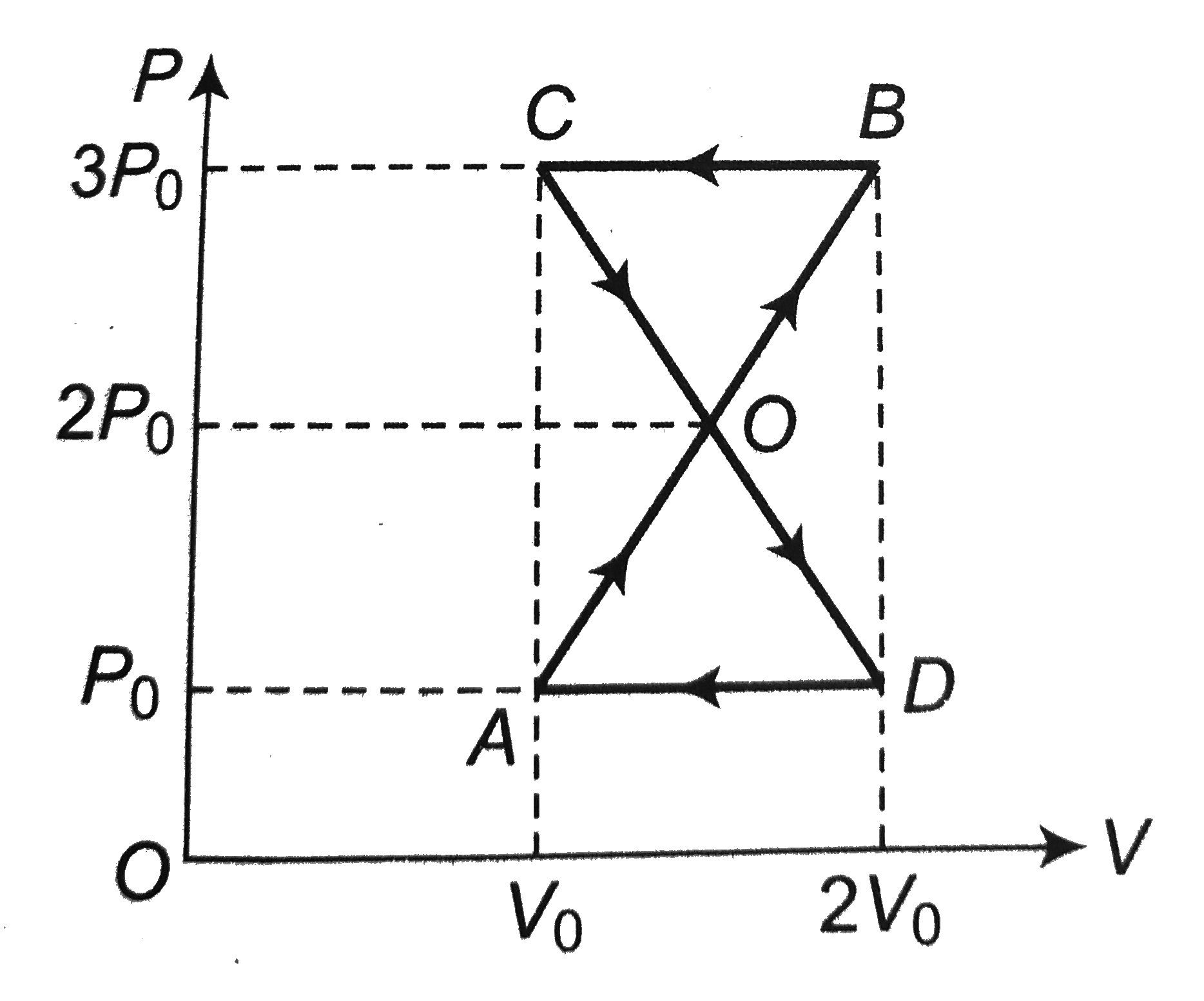
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-LAWS OF THERMODYNAMICS-EXERCISE
- A thermodynamic process is shown in Fig. The pressures and volumes cor...
Text Solution
|
- When a system is taken from state f along path iaf, Q = 50 J and W = 2...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- Consider the cyclic process ABCA, shown in figure, performed on a samp...
Text Solution
|
- The following are the P-V diagrams for cyclic processes for a gas. In ...
Text Solution
|
- In the cyclic process shown in the V-P diagram the magnitude of the wo...
Text Solution
|
- In the previous question (i) work is done by the gas (ii) work is ...
Text Solution
|
- Two different masses m and 3 m of an ideal gas are heated separately i...
Text Solution
|
- A pressure P, absolute temperature T, graph was obtained whe a given m...
Text Solution
|
- A volume V absolute temperature T diagram was obtained when a given ma...
Text Solution
|
- The adjoining figure shows graphs of pressure and volume of a gas at t...
Text Solution
|
- P-V graph was obtained from state 1 to state 2 when a given mass of a ...
Text Solution
|
- A gas expands such that its initial and final temperature are equal . ...
Text Solution
|
- An ideal gas is taken form the state A(P,V) to the state B((P)/(2),2V)...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cyclic process...
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- Six moles of an ideal gas performs a cycle shown in figure, the temper...
Text Solution
|