A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-LAWS OF THERMODYNAMICS-EXERCISE
- A volume V absolute temperature T diagram was obtained when a given ma...
Text Solution
|
- The adjoining figure shows graphs of pressure and volume of a gas at t...
Text Solution
|
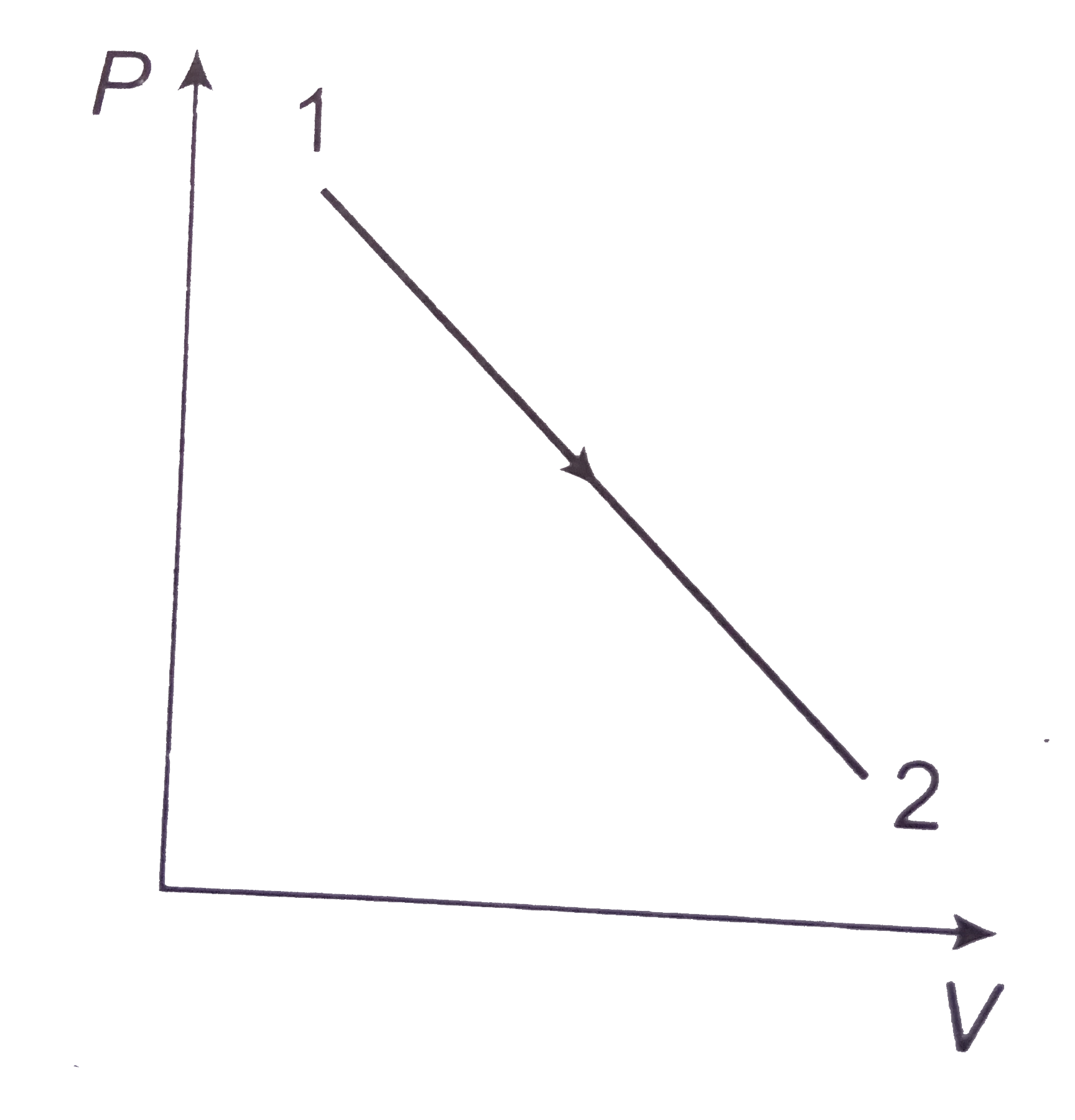
- P-V graph was obtained from state 1 to state 2 when a given mass of a ...
Text Solution
|
- A gas expands such that its initial and final temperature are equal . ...
Text Solution
|
- An ideal gas is taken form the state A(P,V) to the state B((P)/(2),2V)...
Text Solution
|
- One mole of an ideal gas goes from an initial state A to final state B...
Text Solution
|
- One mole of an ideal gas in initial state A undergoes a cyclic process...
Text Solution
|
- Which of the following graphs correctly represents the variation of be...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- Six moles of an ideal gas performs a cycle shown in figure, the temper...
Text Solution
|
- Assertion: The internal energy of an ideal gas does not change during ...
Text Solution
|
- For an isothermal expansion of a perfect gas, the value of (DeltaP)/(P...
Text Solution
|
- If heat is supplied to an ideal gas in an isothermal process.
Text Solution
|
- In an isothermal process on an ideal gas, the pressure increases by 0....
Text Solution
|
- The internal energy of a system remains constant when it undergoes (...
Text Solution
|
- When an ideal gas in a cylinder was compreswsed isothermally by a pist...
Text Solution
|
- During an isothermal expansion, a confined ideal gas does -150 J of wo...
Text Solution
|
- A cyclic process is shown in the P-T siagram. Whech of the curves show...
Text Solution
|
- A cyclic process ABCD is shown is shown in the following P-V diagram. ...
Text Solution
|
- A cyclic process is shown on the P-T diagram. Which of the curve shown...
Text Solution
|