A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-LAWS OF THERMODYNAMICS-EXERCISE
- In case of water from 0 to 4^@C (i) Volume decreases and density of ...
Text Solution
|
- A monoatomic gas of n-moles is heated temperature T1 to T2 under two d...
Text Solution
|
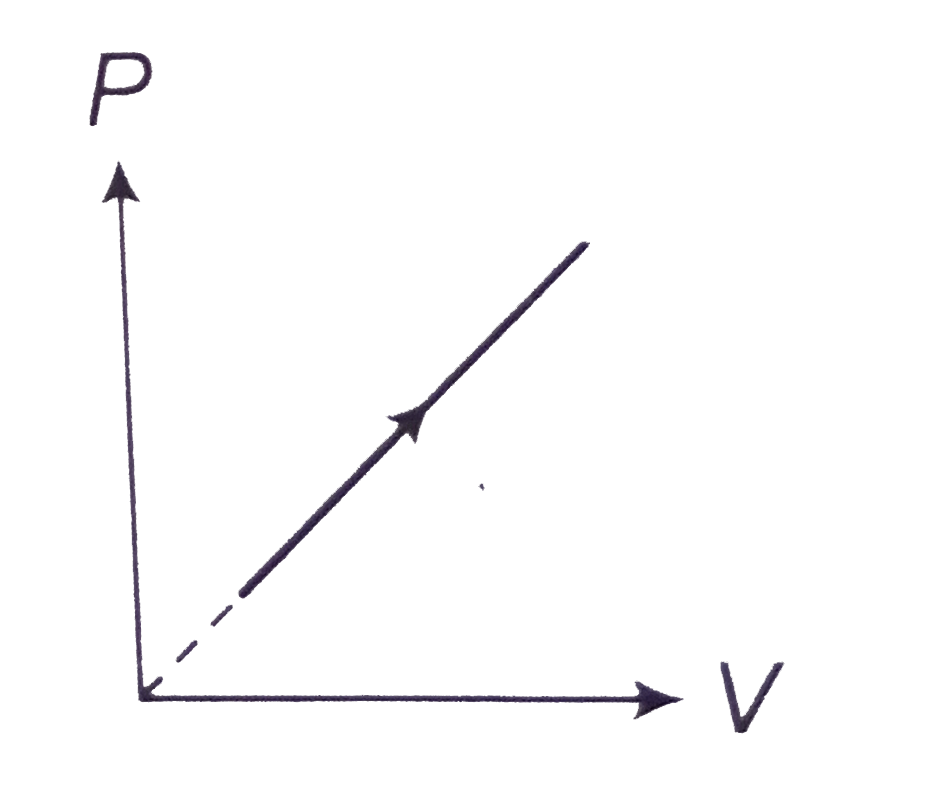
- P-V diagram of a diatomic gas is a straight line passing through origi...
Text Solution
|
- A monoatomic gas is supplied heat Q very slowly keeping the pressure c...
Text Solution
|
- Which of the following is correct regarding adiabatic process (i) In...
Text Solution
|
- Which of the following is correct regarding adiabatic process
Text Solution
|
- The molar heat capacity for an ideal gas (i) Is zero for an adiabatic ...
Text Solution
|
- For an adiabatic expansion of a perfect gas, the value of (DeltaP)/(P)...
Text Solution
|
- In an adiabatic process on a gas with (gamma = 1.4) the pressure is in...
Text Solution
|
- Diatomic gas at pressure 'P' and volume 'V' is compressed adiabaticall...
Text Solution
|
- An ideal gas at 27^(@)C is compressed adiabatically to 8//27 of its or...
Text Solution
|
- An ideal gas at pressure of 1 atmosphere and temperature of 27^(@)C is...
Text Solution
|
- The pressure and density of a diatomic gas (gamma=7//5) change adiabat...
Text Solution
|
- A monoatomic ideal gas, initially at temperature T1, is enclosed in a ...
Text Solution
|
- In an adiabatic change, the pressure p and temperature T of a diatomic...
Text Solution
|
- During an adiabatic process, the pressure of a gas is found to be prop...
Text Solution
|
- The work of 146 kJ is performed in order to compress one kilo mole of ...
Text Solution
|
- The adiabatic elasticity of hydrogen gas (gamma=1.4) at NTP
Text Solution
|
- 1mm^3 of a gas is compressed at 1 atmospheric pressure and temperature...
Text Solution
|
- For which of the following processes is the entropy change zero,
Text Solution
|