A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-LAWS OF THERMODYNAMICS-EXERCISE
- For which of the following processes is the entropy change zero,
Text Solution
|
- If a cylinder containing a gas at high pressure explodes, the gas unde...
Text Solution
|
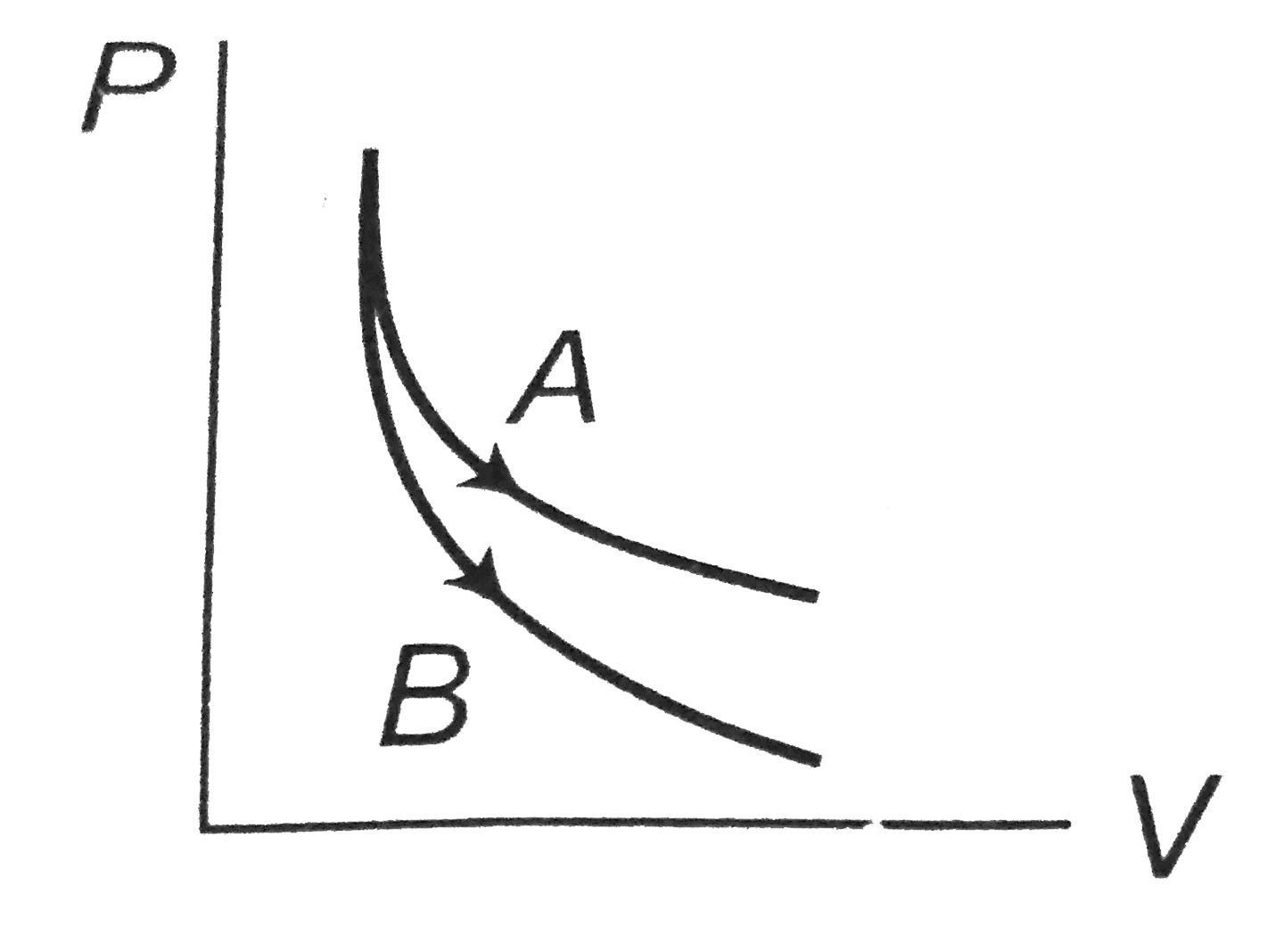
- Consider the process A and B shown in the figure. It is possible that
Text Solution
|
- Two gases have the same initial pressure, volume and temperature. They...
Text Solution
|
- Starting with the same initial conditions, an ideal gas expands from v...
Text Solution
|
- Four curves A, B, C and D are drawn in Fig. for a given amount of gas....
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- Initial pressure and volume of a gas are P and V respectively. First i...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- One mole of an ideal gas at an initial temperature true of TK does 6R ...
Text Solution
|
- If the ratio of specific heat of a gas of constant pressure to that at...
Text Solution
|
- For an ideal gas, (i) the change in internal energy in a constant pr...
Text Solution
|
- A gas may expand either adiabatically or isothermally. A number of p-V...
Text Solution
|
- The internal energy of an ideal gas decreases by the same amount as th...
Text Solution
|
- Three identical adiabatic containers A, B and C Contain helium, neon a...
Text Solution
|
- P-V plots for two gases during adiabatic processes are shown in the fi...
Text Solution
|
- A gas undergoes a process in which its pressure P and volume V are rel...
Text Solution
|
- In the figure shown, the processes leveled 1,2,3 and 4 are
Text Solution
|
- "Heat cannot by itself flow from a body at lower temperature to a body...
Text Solution
|
- A carnot cycle has the reversible process in the following order:
Text Solution
|