A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-LAWS OF THERMODYNAMICS-EXERCISE
- P-V plots for two gases during adiabatic processes are shown in the fi...
Text Solution
|
- A gas undergoes a process in which its pressure P and volume V are rel...
Text Solution
|
- In the figure shown, the processes leveled 1,2,3 and 4 are
Text Solution
|
- "Heat cannot by itself flow from a body at lower temperature to a body...
Text Solution
|
- A carnot cycle has the reversible process in the following order:
Text Solution
|
- Choose the incorrect statement from the following: S1: The efficienc...
Text Solution
|
- An ideal gas is subjected to cyclic process involving four thermodynam...
Text Solution
|
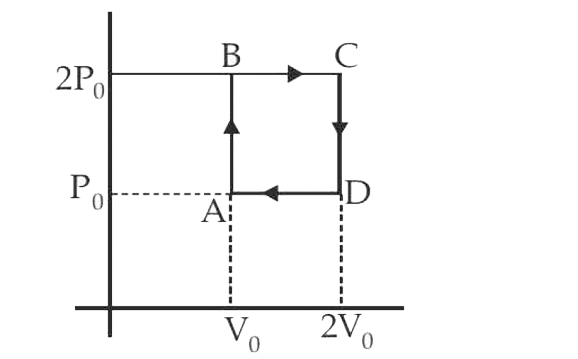
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- The maximum possible efficiency of an engine that aborbs hat at 327^@...
Text Solution
|
- The efficiency of a carnot engine is (1)/(6). If the temperature of th...
Text Solution
|
- Efficiency of a Carnot engine is 50% when temperature of outlet is 500...
Text Solution
|
- An ideal refrigerator has a freezer at a temperature of -13^(@)C. The ...
Text Solution
|
- An ideal refrigerator is used to transfer heat from a freezer at -23^@...
Text Solution
|
- In a refrigerator, heat from inside at 277K is transferred to a room a...
Text Solution
|
- If the door of a refrigerator is kept open, then which of the followin...
Text Solution
|
- A Carnot engine, having an efficiency of eta=1//10 as heat engine, is ...
Text Solution
|
- Which of the following statements is correct for any thermodynamic sys...
Text Solution
|
- A mearsure of the degree of disorder of a system is known as
Text Solution
|
- The change in the entropy of a 1 mole of an ideal gas which went throu...
Text Solution
|
- If heat Q is added reversibly to a system at temperature T and heat Q'...
Text Solution
|