Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CP SINGH-THERMAL AND ELECTRIC EFFECT oF CURRENT-Exercises
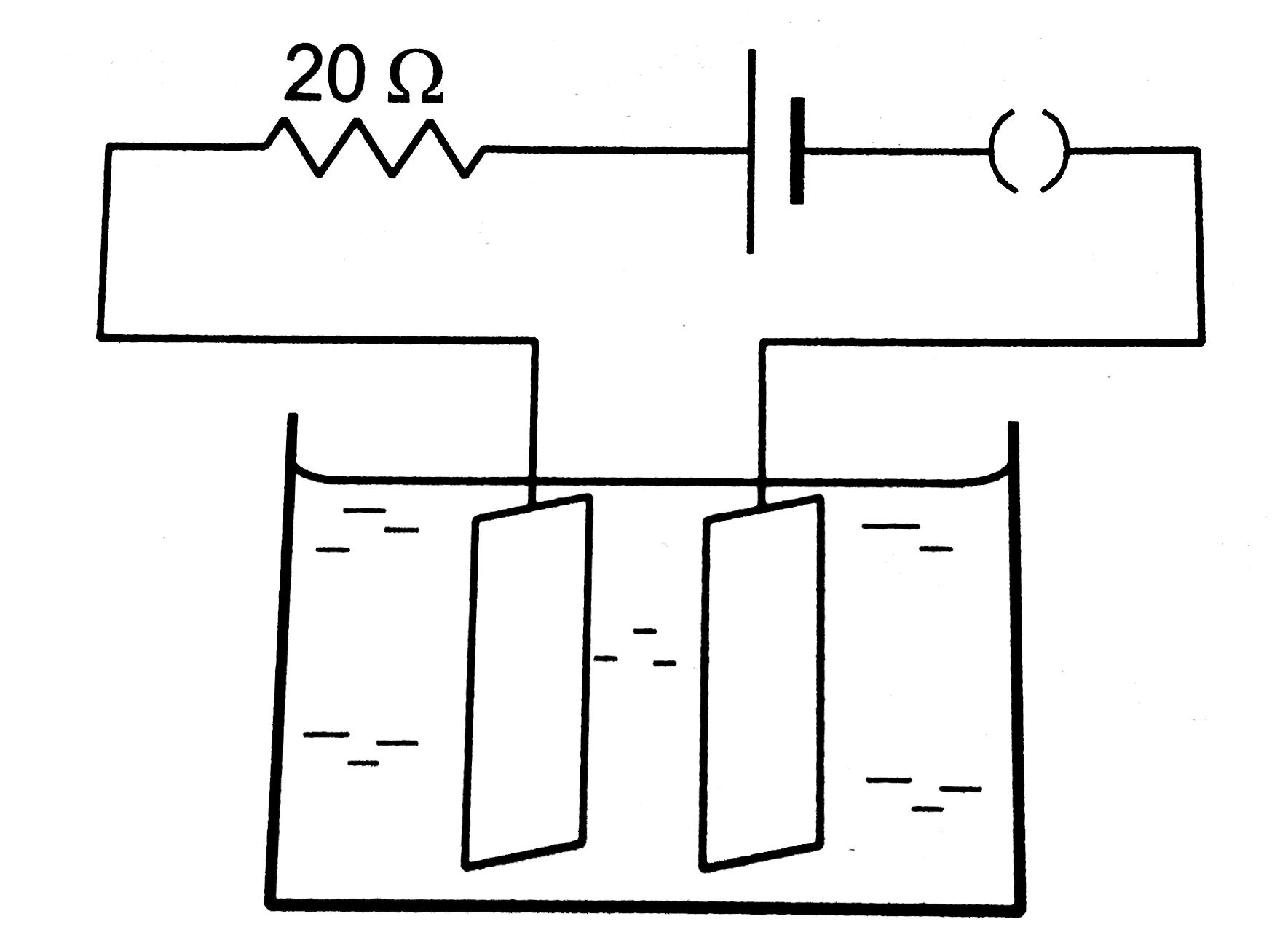
- Figure, shows an electrolyte of AgCI through which a current is passed...
Text Solution
|
- According to Joule's law, if the potential difference across a conduct...
Text Solution
|
- If the current in an electric bulb drops by 1% the power decreases by
Text Solution
|
- (a) When a cell sends current through a resistance R(1) for time t, th...
Text Solution
|
- Two resistances , connected in parallel across a source of negligible ...
Text Solution
|
- A heating coil transforms 100J of electrical energy into heat energy p...
Text Solution
|
- A constant voltage is applied between the two ends of a uniform metall...
Text Solution
|
- You are given a resistance coil and a battery. In which of the followi...
Text Solution
|
- Two indential heated produce heat H(1) in time t when connected in par...
Text Solution
|
- A 500W heating unit is designed to operate from a 115 "volt" line. If...
Text Solution
|
- Three equal resistor connected in series across a source of enf toget...
Text Solution
|
- A current of 3 A flows through the 2 Omega resistor as shown in the ci...
Text Solution
|
- A heater coil is cut into two parts of equal length and one of them is...
Text Solution
|
- The three resistance of equal value are arranged in the different comb...
Text Solution
|
- For ensuring dissipation of same energy in all three resistors (R(1), ...
Text Solution
|
- A wire of length L and 3 identical cells of negligible internal resist...
Text Solution
|
- An electric kettle has two coils. When one coil is switched an it tak...
Text Solution
|
- An electric kettle has two heating coils. when one coil is used, water...
Text Solution
|
- An electric kettle of 1200Whas 1 kg water at 20^@C. The water equivale...
Text Solution
|
- Water of volume 2 litre in a container is heated with a coil of 1kW at...
Text Solution
|
- The power dissipated 6 Omega resistor is
Text Solution
|