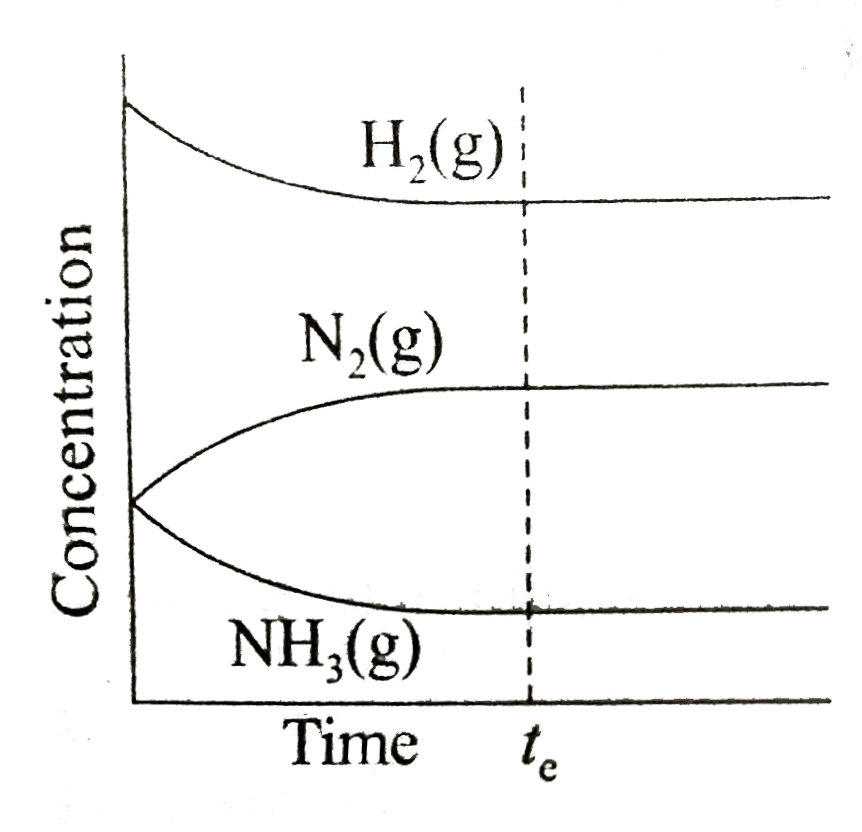
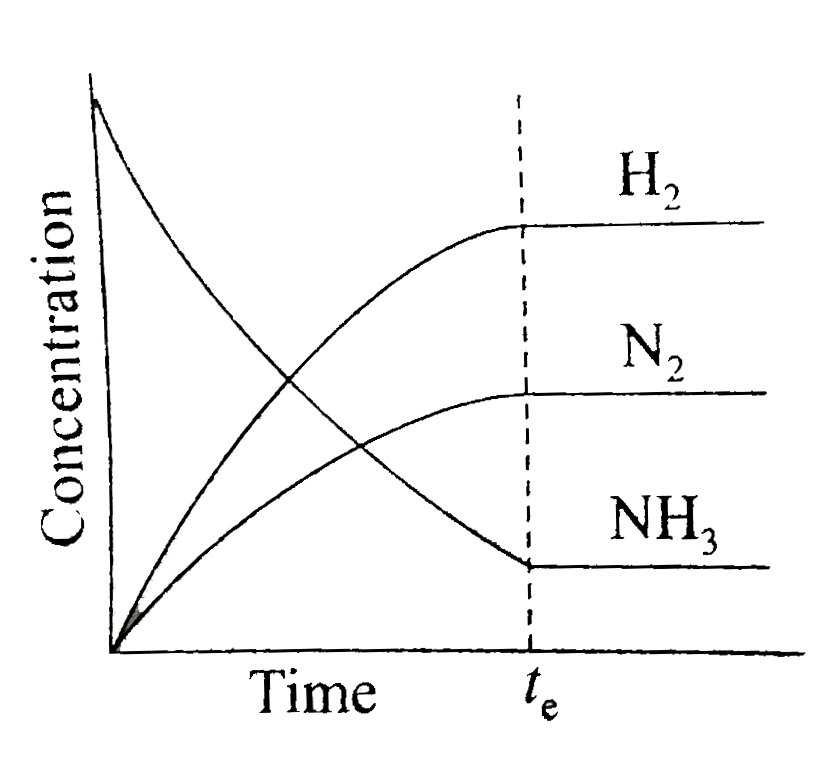
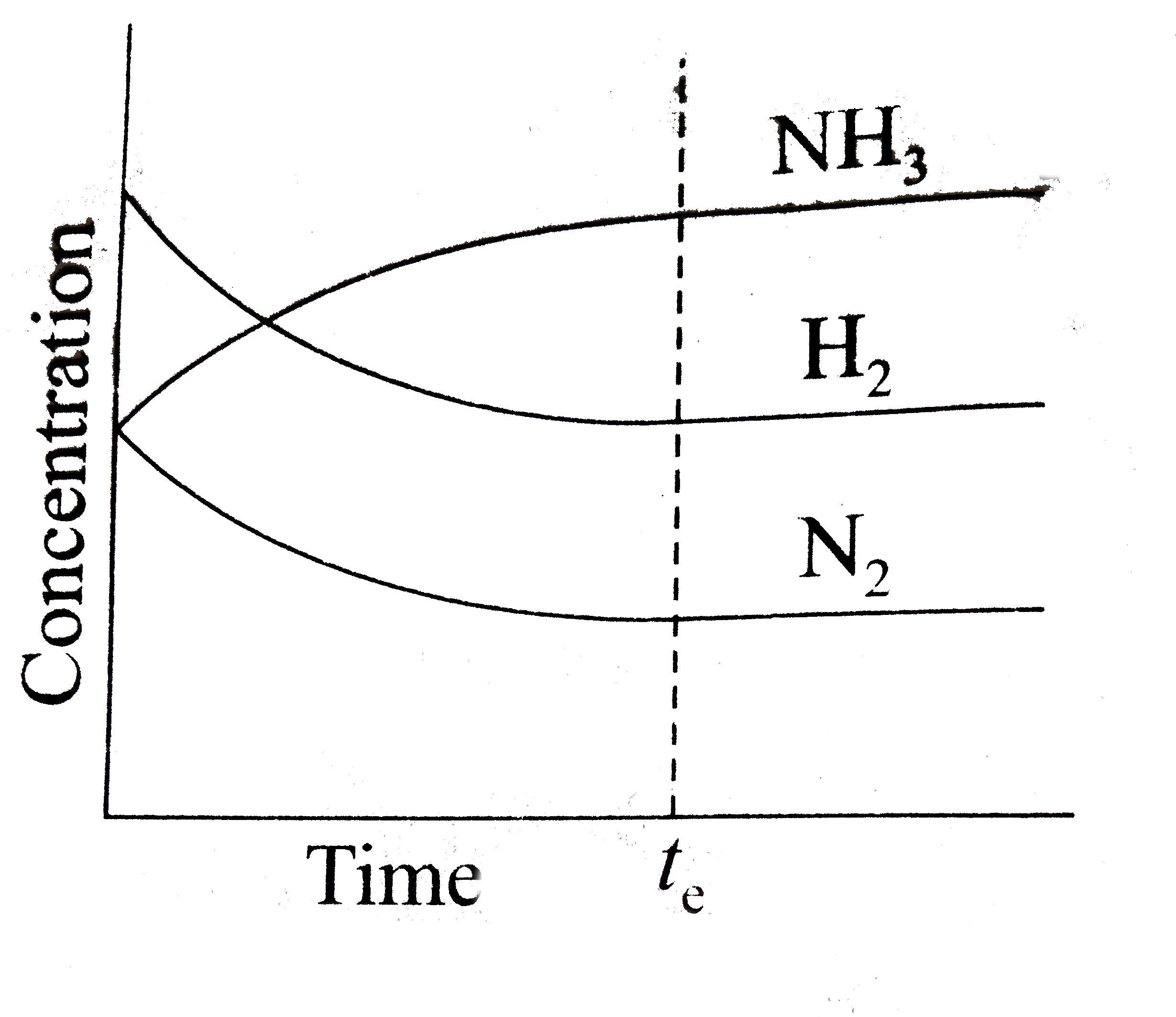
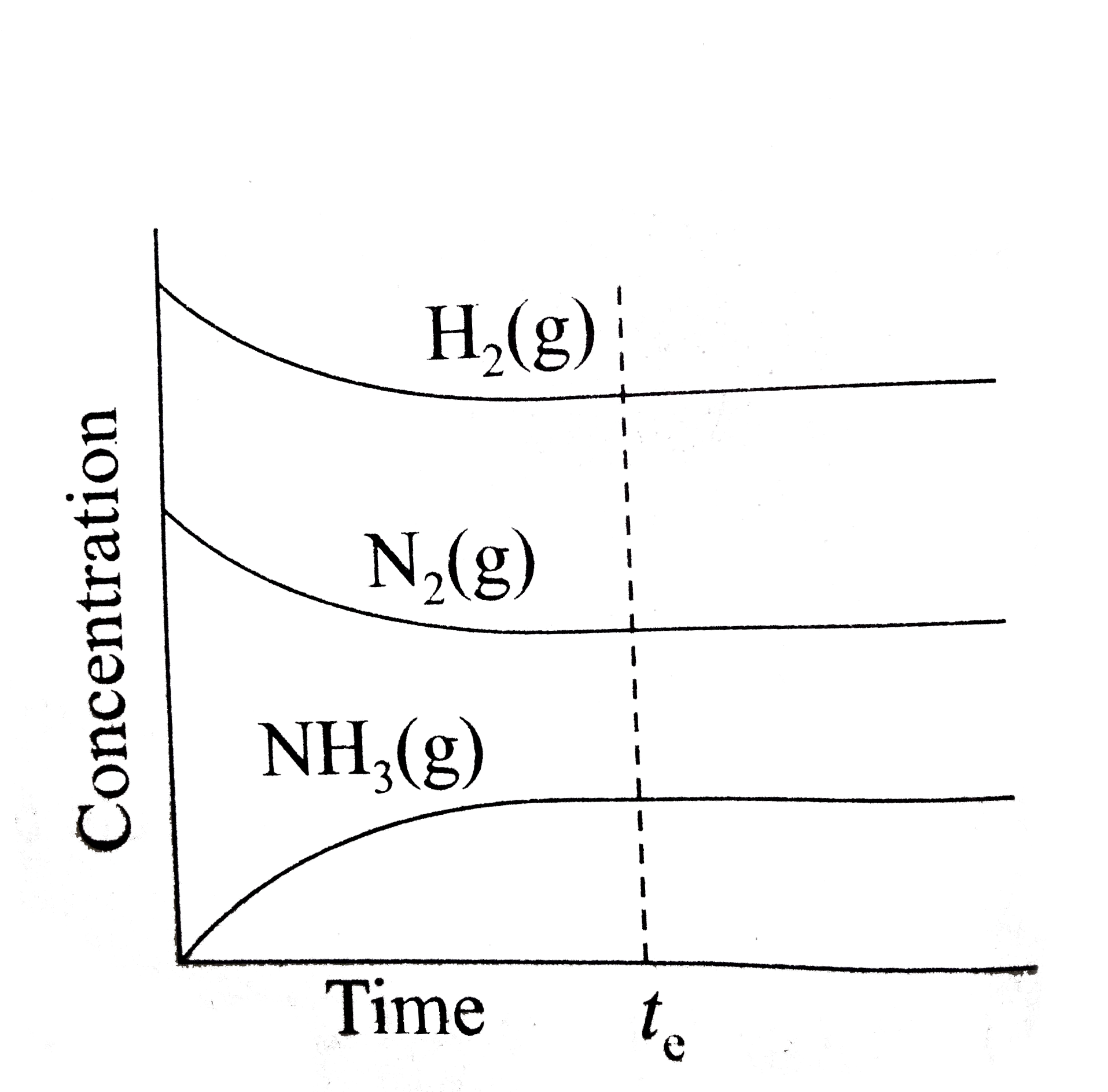A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
R SHARMA|Exercise Follow-up Test 3|13 VideosEQUILIBRIUM
R SHARMA|Exercise Follow-up Test 4|10 VideosEQUILIBRIUM
R SHARMA|Exercise Follow-up Test 1|10 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Archives|36 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-EQUILIBRIUM-Follow-up Test 2
- A reversible chemical reaction is said to be at equilibrium when
Text Solution
|
- Chemical equilibrium is a dynamic equilibrium because
Text Solution
|
- An example of a reversible reaction is
Text Solution
|
- The reaction which proceeds in the forward direction is.
Text Solution
|
- Which of the following correctly depicts the attainment of equilibrium...
Text Solution
|
- Equilibrium mixture (I) consists of N2, H2, and NH3. Equilibrium mixtu...
Text Solution
|
- Which of the following correctly depicts the fact that identical chemi...
Text Solution
|