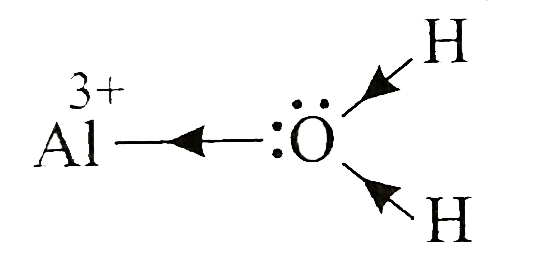
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
EQUILIBRIUM
R SHARMA|Exercise Follow-up Test 13|10 VideosEQUILIBRIUM
R SHARMA|Exercise Follow-up Test 14|12 VideosEQUILIBRIUM
R SHARMA|Exercise Follow-up Test 11|16 VideosCLASSIFICATION OF ELEMENTS AND PERIODICITY IN ELEMENTS
R SHARMA|Exercise ARCHIVES|37 VideosGENERAL ORGANIC CHEMISTRY
R SHARMA|Exercise Archives|36 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-EQUILIBRIUM-Follow-up Test 12
- The pH of a solution containing 0.20M CH(3)COOH and 0.30M CH(3)COONa i...
Text Solution
|
- When CH(3)COONa is added to an aqueous solution of CH(3)COOH
Text Solution
|
- The pK(a) of acteylsalicylic acid (aspirin) is 3.5. The pH of gastric ...
Text Solution
|
- 50.0 mL of 0.10 M ammonia solution is treated with 25.0 mL of 0.10M HC...
Text Solution
|
- Which of the following cations is not hydrolyzed in aqueous solution ?...
Text Solution
|
- Which of the anions is not hydrolyzed in aqueous solution ? CI^(-) ...
Text Solution
|
- Which of the following salts does not undergo hydrolysis ?
Text Solution
|
- Which of the following salts undergoes anionic hydrolysis ?
Text Solution
|
- The aqueous solution of aluminium chloride is acidic due to the
Text Solution
|
- Which of the following relations is correct during the hydrolysis of s...
Text Solution
|
- For the aqueous solution of a salt of a weak acid abd a weak base,
Text Solution
|
- For cationic hydrolysis, pH given by
Text Solution
|
- Which of the following salts is neutral in water ?
Text Solution
|