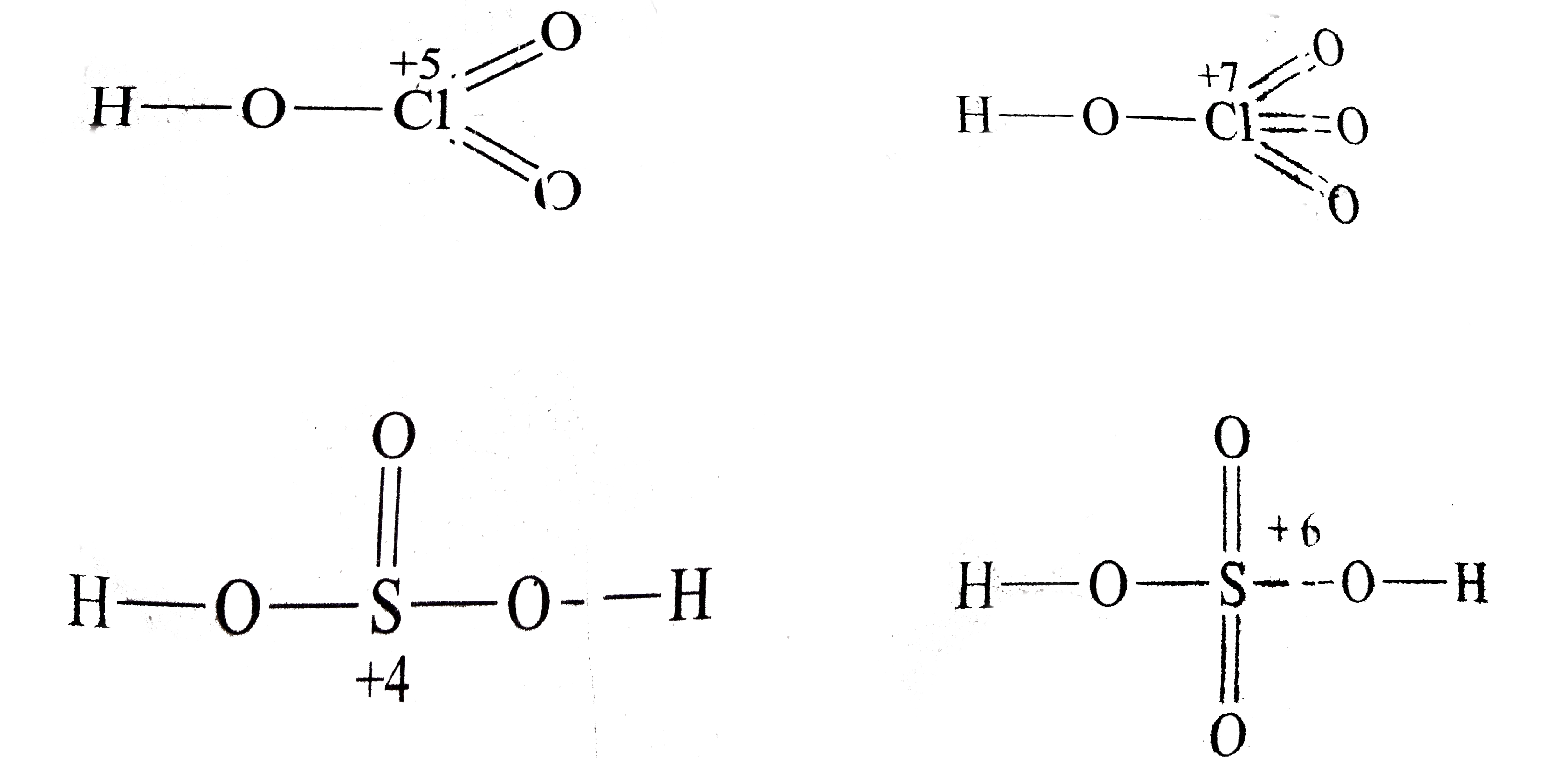
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-EQUILIBRIUM-ARCHIVES
- Indentify the correct order of solubility in aqueous medium
Text Solution
|
- Which of these is least likely to act as Lewis base?
Text Solution
|
- Which is the strongest acid in the following ?
Text Solution
|
- The dissociation constants for acetic acid and HCN at 25^(@)C are 1.5x...
Text Solution
|
- What is the [OH^(-)] in the final solution prepared by mixing 20.0 mL ...
Text Solution
|
- The ionization constant of ammonium hydroxide is 1.77xx10^(-5) at 298 ...
Text Solution
|
- Which of the following molecules acts as a Lewis acid?
Text Solution
|
- Equimolar concentrations of H(2) and I(2) are heated to equilibrium in...
Text Solution
|
- The number of H^(+) ions present in 250 ml of lemon juice of pH=3 is
Text Solution
|
- The values of K(p) and Kp(2) fot the reactions XhArrY+Z , (a) an...
Text Solution
|
- The dissociation equilibrium of a gas AB(2) can be represented as 2A...
Text Solution
|
- If the concentration of OH^(-) ions in the reaction Fe(OH)(3)(s)hArr...
Text Solution
|
- Equimolar solution of the following were prepared in water separately....
Text Solution
|
- Equal volumes of three acid solutions of pH 3, 4 and 5 are mixed in a ...
Text Solution
|
- The equilibrium constant (K(p)) for the decomposition of gaseous H(2)O...
Text Solution
|
- The aqeous solutions of HCOONa, C(6)H(5)NH(3)CI, and KCN are, respecti...
Text Solution
|
- Which one of the following ionic speeies has the greatest protonaffini...
Text Solution
|
- A weak acid, HA, has a K(a) of 1.00xx10^(-5). If 0.100 mol of the acid...
Text Solution
|
- Calculate the pOH of solution at 25^(@)C that contains 1xx10^(-10) M o...
Text Solution
|
- When hydrogen molecules decompose into its atoms, which conditions giv...
Text Solution
|