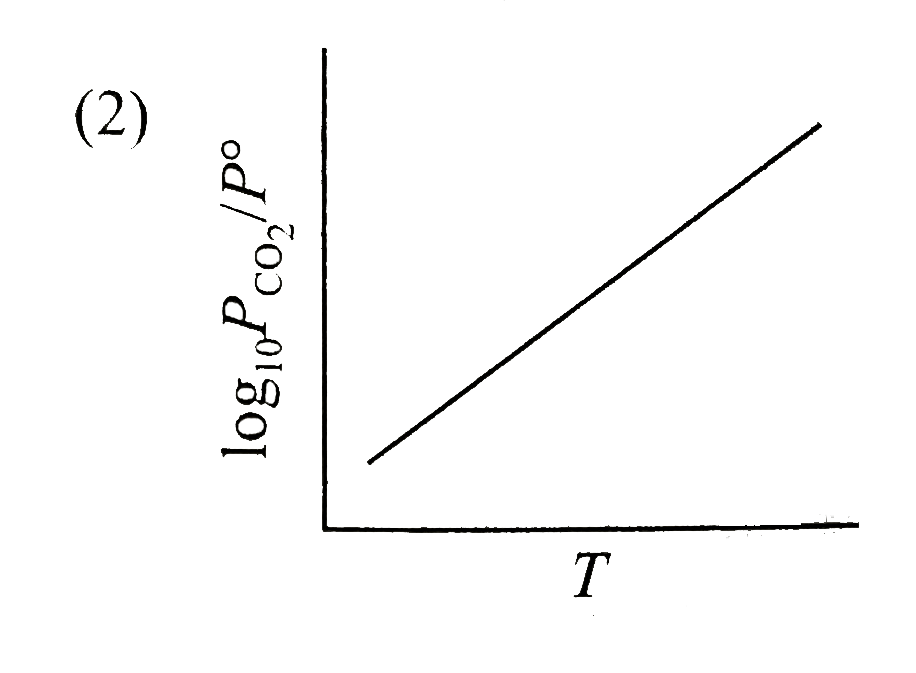
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-EQUILIBRIUM-ARCHIVES
- NH(4)COONH(2)(s)hArr2NH(3)(g)+CO(2)(g) If equilibrium pressure is 3 at...
Text Solution
|
- A + B hArrC + D. If finally the concentrations of A an d B are both eq...
Text Solution
|
- For the chemical equilibrium, CaCO(3)(s) hArr CaO(s)+CO(2)(g) Delt...
Text Solution
|
- Equilibirum constants K(1) and K(2) for the following equilibria NO(...
Text Solution
|
- Which of the following anions is the weakest base ?
Text Solution
|
- A solution has pH=5, it is diluted 100 times, then it will become
Text Solution
|
- The K(sp) of Mg(OH)(2) is 1xx10^(-12). 0.01M Mg^(2+) will precipitate ...
Text Solution
|
- At 25^(@)C, the dissociation constant of a base. BOH is 1.0xx10^(-12)...
Text Solution
|
- When 10 ml of 0.1 M acitec acid (pk(a)=5.0) is titrated against 10 ml ...
Text Solution
|
- H(2)S gas when passed through a solution of cations containing HCl pre...
Text Solution
|
- The correct order of acid strength is
Text Solution
|
- What is the correct relationship between the pH of isomolar solutions ...
Text Solution
|
- 2 mol of N(2) is mixed with 6 mol of H(2) in a closed vessel of 1L cap...
Text Solution
|
- Ammoina carbonate when heated to 200^(@)C gives a mixture of NH(3) and...
Text Solution
|
- A mixture of NO(2) and N(2)O(4) has a vapor density of 38.3 at 300 K. ...
Text Solution
|
- Of the following, which change will shift the reaction towards the pro...
Text Solution
|
- What will be the pH of 0.05M barium hydroxide solution ?
Text Solution
|
- The only cations present in a slightly acidic are Fe^(3+), Zn^(2+), an...
Text Solution
|
- The principal buffer present in human blood is
Text Solution
|
- 40 mg of pure sodium hydroxide is dissolved in 10 L of distilled water...
Text Solution
|