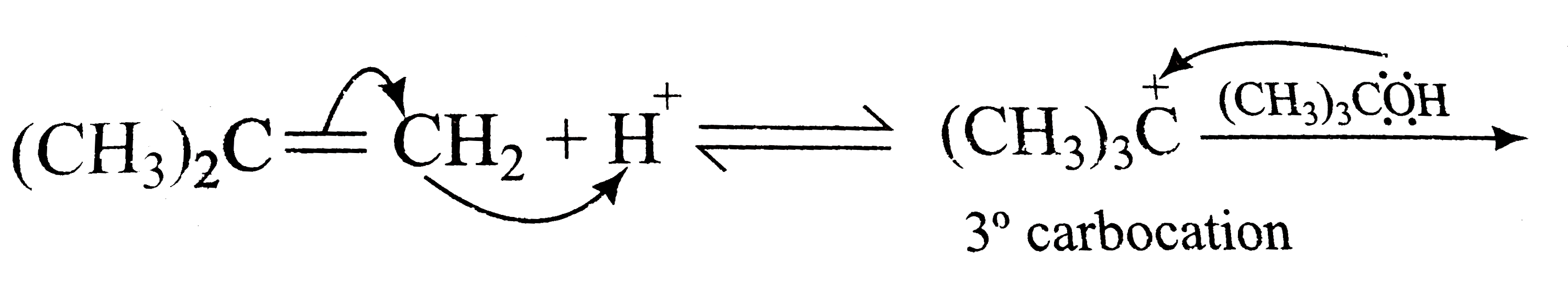
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -13
- Identify the final product (B) in the following sequence of reactions....
Text Solution
|
- Consider the following reactions CH(3)CH=CH(2)overset(1.(CH(3)COOH)(...
Text Solution
|
- Which of the following is not expected to produce methoxybenzene?
Text Solution
|
- Reaction of t-butyl bromide with sodium methoxide produces
Text Solution
|
- In the reaction
Text Solution
|
- Which of the following is not expected to give ether on reaction with ...
Text Solution
|
- Ethyl bromide on heating with dry silver oxide gives
Text Solution
|
- Phenol, C(6)H(5)OH is allowed to react with diazomethane, CH(2)N(2). T...
Text Solution
|
- Ethyl alcohol (in excess) is heated with concentrated H(2)SO(4) at 40^...
Text Solution
|
- The reaction 2C(2)H(5)Ohoverset(Conc.H(2)SO(4))underset(140^(@)C)(ra...
Text Solution
|
- Di-t-butyl ether is prepared best by the reaction
Text Solution
|
- A mixture of CH(3)OH and C(2)H(5)OH is heated with concentrated H(2)SO...
Text Solution
|
- Which of the following reactions is the best choic for preparing methy...
Text Solution
|
- Consider the following reactions CH(3)CH=CH(2)overset(HBr)underset((...
Text Solution
|
- In the reaction sequence The product (B) is
Text Solution
|