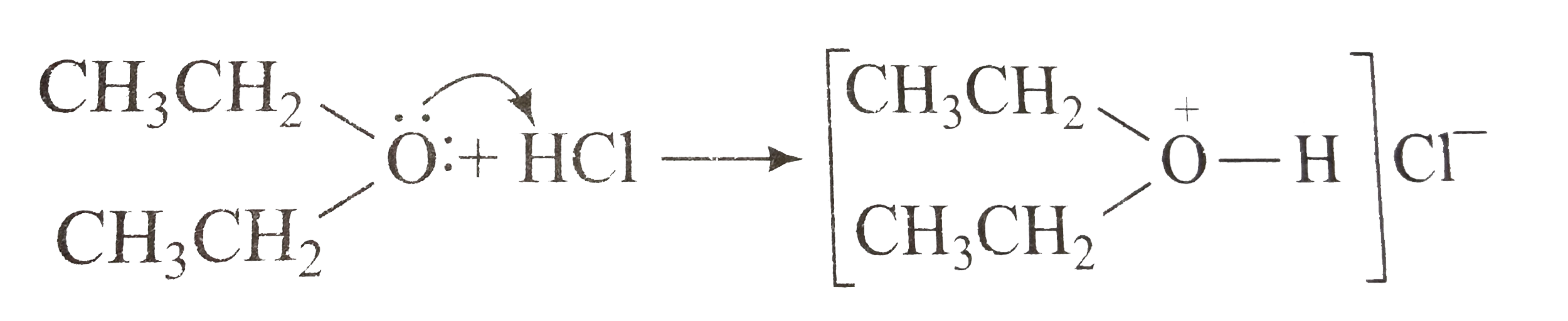
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOL, PHENOL AND ETHERS
R SHARMA|Exercise Quation Bank(Building the knowledge) Level I|7 VideosALCOHOL, PHENOL AND ETHERS
R SHARMA|Exercise Quation Bank(Building the knowledge) Level II|28 VideosALCOHOL, PHENOL AND ETHERS
R SHARMA|Exercise Follow -Up Test -13|15 VideosAMINES
R SHARMA|Exercise Archives|36 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Follow -Up Test -14
- The reaction of C(2)H(5)OC(2)H(5) with BF(3) leads to the formation of
Text Solution
|
- Which of the following is a very good ethylating agent for converting ...
Text Solution
|
- Diether ether becomes soluble in concentrated HCl in cold condition du...
Text Solution
|
- The product of the reaction is
Text Solution
|
- In the reaction (CH(3))(3)C-O-CH(2)CH(3)+underset((1"mole"))(HI)over...
Text Solution
|
- Which of the following ethers is the most unreactive to cleavage with ...
Text Solution
|
- Diethyl ether on reaction with CI(2) in the dark at room temperature f...
Text Solution
|
- Diethyl ether on prolonged exposure to air forms
Text Solution
|
- Ethyl methyl ether on heating with PCl(5) produces
Text Solution
|