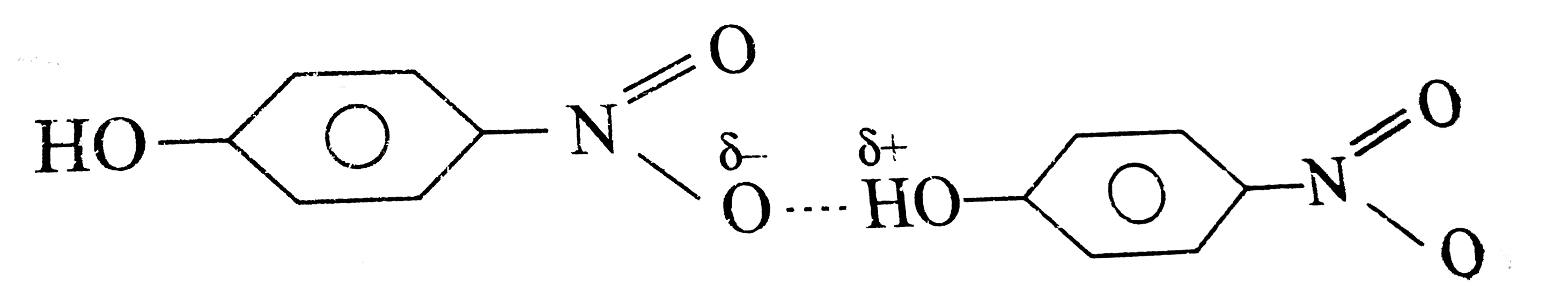A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-PHENOLS (II)
- When phenol is heated with CHCl(3) and alcoholic KOH, salicylaldehyde ...
Text Solution
|
- Increasing order of acidic strength among p-methoxyphenol (i)p-methylp...
Text Solution
|
- The boiling point of p-nitrophenol is higher than that of o-nitropheno...
Text Solution
|
- Isopropylbenzene on air oxidation in the presence of dilute acid gives
Text Solution
|
- Phenol can be distinguished from ethanol by the following reagents exc...
Text Solution
|
- The compound used to manufacture phenol is
Text Solution
|
- Salicylic acid is prepared from phenol by
Text Solution
|
- Picric acid is
Text Solution
|
- Na reacts with phenol to produce
Text Solution
|
- Zinc powder+PhOHrarrX In the above reaction the product X will be
Text Solution
|
- Which of the following statements is correct?
Text Solution
|
- Electophilic substitution in phenol generally occurs at
Text Solution
|
- In order to get Bakelite from phenol which of the following reagents i...
Text Solution
|
- Which of the following groups will increasing the acidity of phenol?
Text Solution
|
- Among the following phenols which is most acidic?
Text Solution
|
- Salicyladehyde can be prepard form
Text Solution
|
- Phenol is heated with C Cl(4) and alkaline KOH when salicylic acid is ...
Text Solution
|
- Phenol on treatment with conc. HNO(3) gives
Text Solution
|
- C(6)H(5)Ohoverset(CH(3)COCl)underset(NaOH(aq))(rarr)C(6)H(5)OCOCH(3) ...
Text Solution
|
- When phenol is treated with excess of bromine water, it gives
Text Solution
|