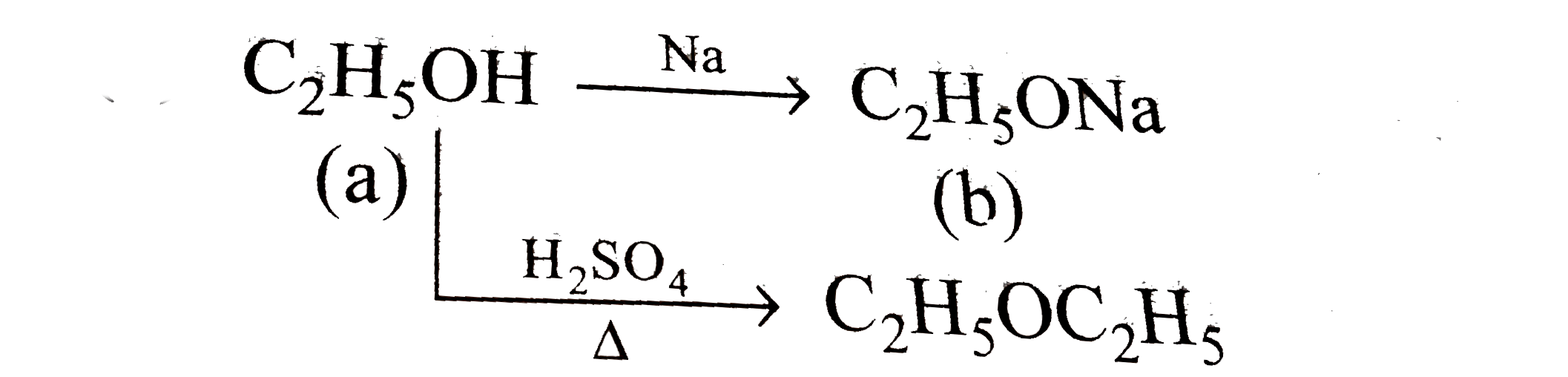A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-ETHERS level I
- The reaciton of an alkyl halide with a metal alkoxide forming an ether...
Text Solution
|
- Which of the following is not an isomer of diethyl ether?
Text Solution
|
- An organic compound (a) reacts with sodium metal and forms (b). On hea...
Text Solution
|
- The reaciton C(2)H(5)Ona+C(2)H(5)I-C(2)H(5)OC(2)H(5)+NaI is an ex...
Text Solution
|
- Which of the following is a symmetrical ether?
Text Solution
|