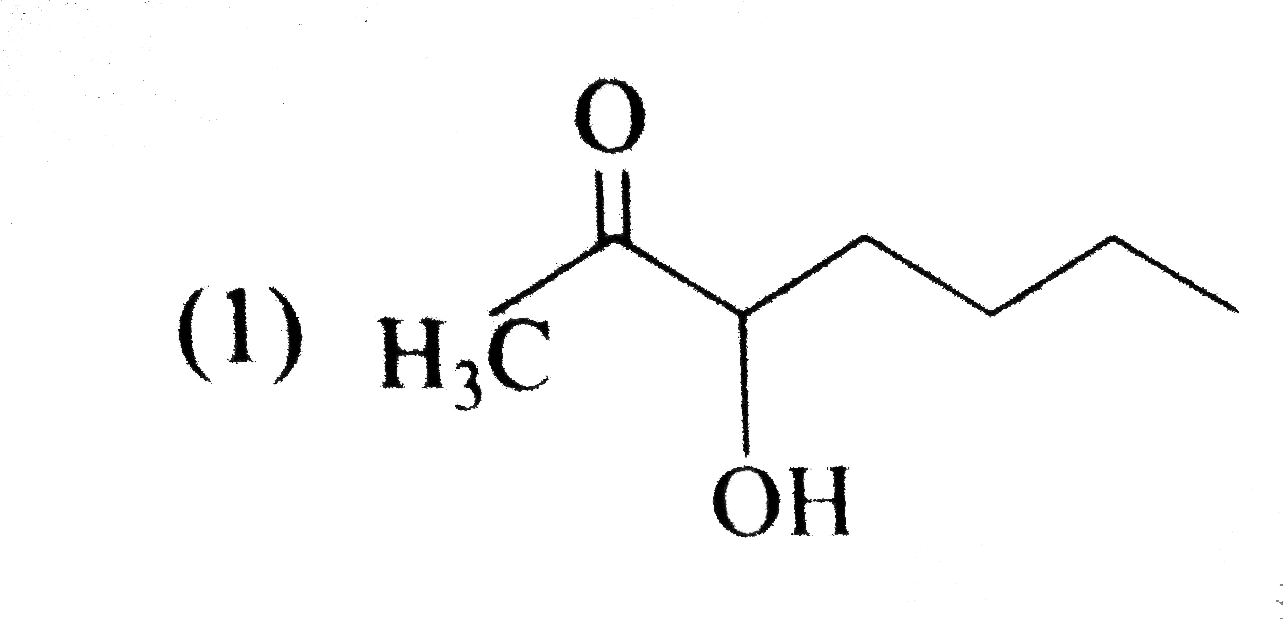
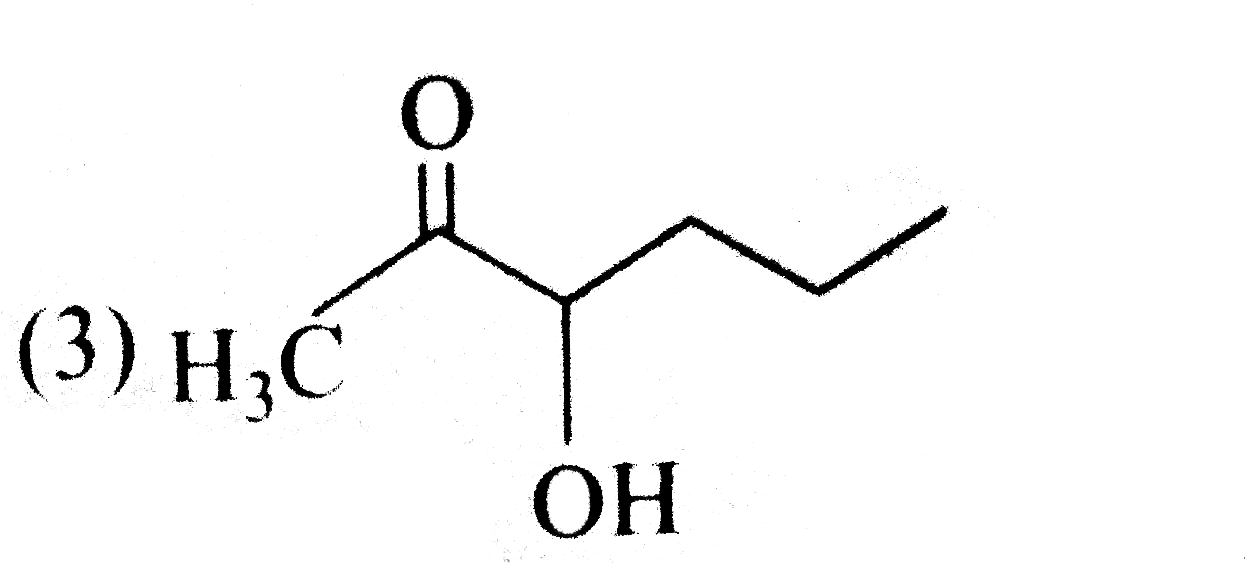
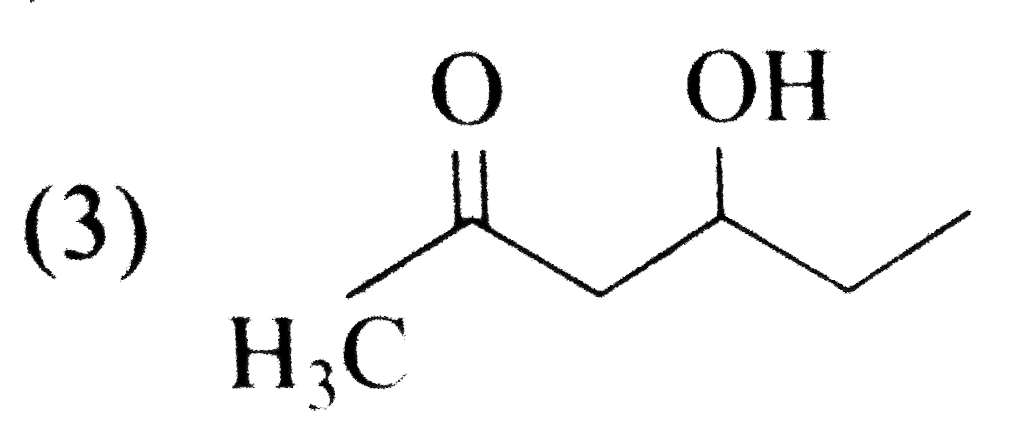
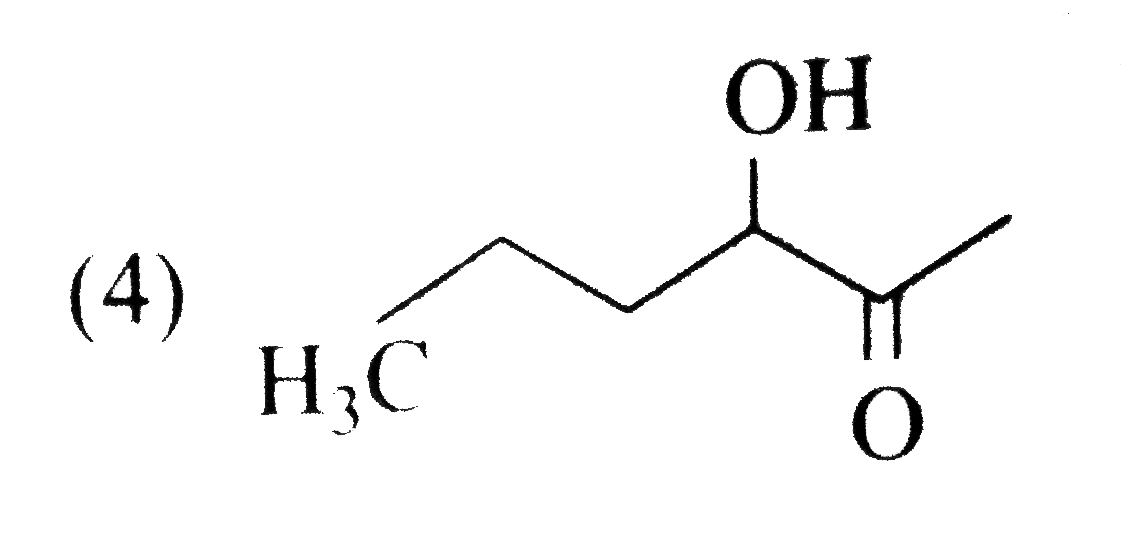
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-ALCOHOL, PHENOL AND ETHERS-Archives
- Given are cyclohexanol (I), acetic acid (II),2,4,6-trinitrophenol (III...
Text Solution
|
- Among the following four compounds
Text Solution
|
- Which one of the following compounds will be most readily dehydrated?
Text Solution
|
- Which of the following conformers for ethylene glycol is most stable?
Text Solution
|
- Following compounds are given (a) CH(3)CH(2)OH , (b) CH(3)COCH(3) ...
Text Solution
|
- Glycerol on being heated with an excess of HI produces
Text Solution
|
- Consider the following reaction ethanol overset(PBr(3))(rarr)Xoverse...
Text Solution
|
- Consider the following reaction Phenol overset(Zn)underset("dust")ra...
Text Solution
|
- CH(2)OHCH(2)OH on heating with periodic acid gives
Text Solution
|
- In the reaction CH(3)overset(CH(3))overset(|)(C )H-CH(2)-O-CH(2)CH(3...
Text Solution
|
- The major organic product in the reaction CH(3)-O-CH(CH(3))(2)+Hirar...
Text Solution
|
- The general molecular formula, which represents the homologous series ...
Text Solution
|
- Ethylene oxide when treated with Grignard reagent yields
Text Solution
|
- Which one of the following compounds is most acidic
Text Solution
|
- The enzyme which hydrolyses triglycerides to fatty acid and glycerol i...
Text Solution
|
- Which of the following will not form a yellow precipitate on heating w...
Text Solution
|
- The -OH group of an alcohol or of the -COOH group of a carbocylic acid...
Text Solution
|
- Which of the following orders of acid strength is correct?
Text Solution
|
- When phenol is treated with CHCl(3) and NaOH, the product fromed is
Text Solution
|
- n-propyl alcohol can be chemical distinguished by which reagent
Text Solution
|