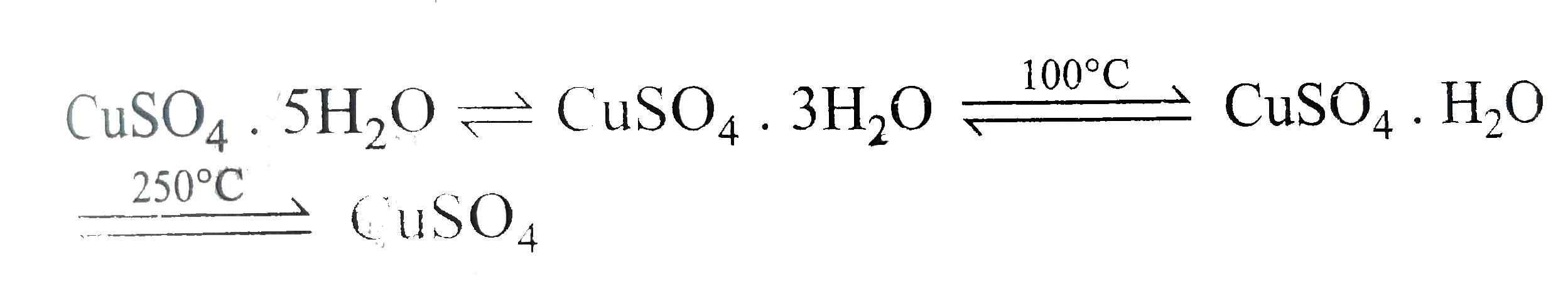A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-SOLUTIONS-FOLLOW-UP TEST 1
- Which of the following statements is incorrect about a solution?
Text Solution
|
- Carat is a measure of diamonds and other gems, formerly 3.17 grains (0...
Text Solution
|
- 18 carat gold contains 18 parts in 24 parts and has a fineness of
Text Solution
|
- CuSO(4).5H(2)O, a hydrated salt, is a coordination compound of Cu^(2+)...
Text Solution
|
- Which of the following represents a metastable system?
Text Solution
|