Octane is a nonpolar hydrocarbon found in gasoline while water is a highly polar hydrogen-bonding solvent. Octane can't from hydrogen bonds, so there is no good way for the molecules of these two substances to intract strongly with each other.
There are no favorable solute-solvent interactions to compensate for the solute-solute attractions and solvent-solvent attractions that must be overcome if the solute is to disperse in the solvent. Thus, octane and water are not miscible.
II. Acetone is an organic solvent. Its molecule, `CH_(3)-overset(O)overset(||)(C)-CH_(3)`, has a relatively negative oxygen atom that can form a hydrogen bond with the hydrogen of water
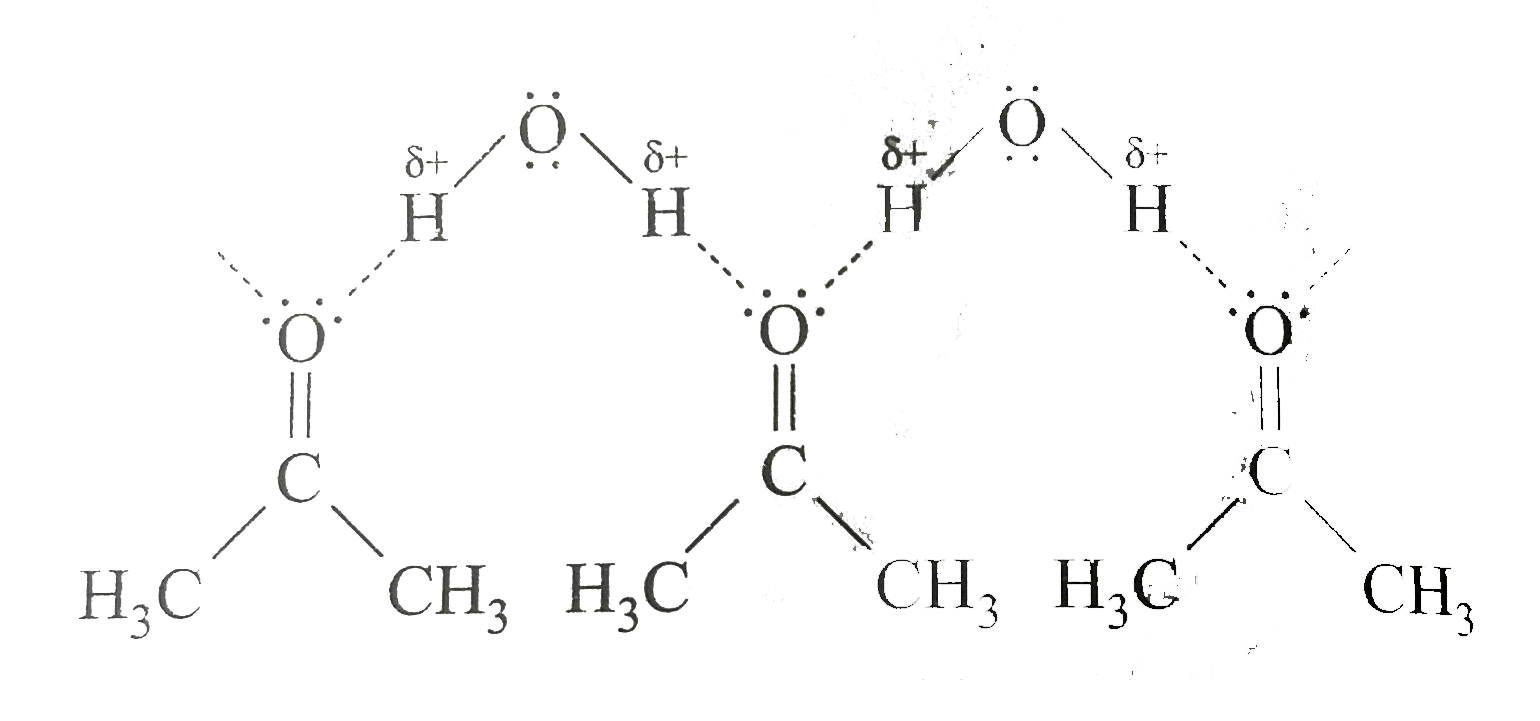
Thus, water and acetone are miscible. Acetone and octane are also miscible because the interactions between molecules of octane and acetone are similar in magnitude to those between molecules of acetone or of octane alone, since aceton can't form hydrogen bonds with it self. When acetone and octane are mixed there is nothing to prevent the molecules of the two substances from mixing freely.
III. Lithum chloride (LiCl) is an ionic, highly polar salt. It is most soluble (about ` 80 g//100 g H_(2)O)` in water, the polar solvent for several reasons. The attractive foeces between the anions and the cations of LiCl are reduced in water because of the high dielectric constant of water. In addition, ion-dipole attractions occure between the `Li^(+)` cations and the relatively negative oxygen stoms of the water molecules. Hydrogen bonding between the `Cl^(-)` anions and the water molecules also occures. These favorable solute-solvent interactions more than compensate for the loss of the attractions in pure water and pure `LiCl` (i.e. an ionic solid dissolves in water because the attractions between the polar molecules and the ions making up the crystal lattice of the solid are greater than the attractive forces that hold the lattice together).
LiCl is less soluble in acetone (about ` 5g//100g of "acetone")`. The acetone molecules do not interact as favorably with dissolved ions as do `H_(2)O` molecules, and acetone has a lower dieldctric constant than water.
Finally, LiCl does not dissolve at all in acetone, because there are no favorable interactions between the charged ions of `LiCl` and the nonpolar molecules of octane.
The behavior of a nonpolar hydrocarbon solute such as naphthalene, `C_(10)H_(8)` (mothballs) is opposite to that of an ionic salt. Naphthalene, a solid dissolves in octane becomes the interactions between molecules of naphthalene and molecules of octane are similar in magnitude to those between molecules of naphalene alone or of octane alone.
Naphthalene is less soluble is acetone as there are some polar attractions between molecules of acetone, and naphthalene does not interact well enough will acetone to compensate for the loss of these attractions.
Naphthlene is virtually insolublr in water. There are no favorable attractions between water molecules and non-polar naphthalene molecules to compensate for the energy required to break the strong hydrogen bonds between the water molecules.
