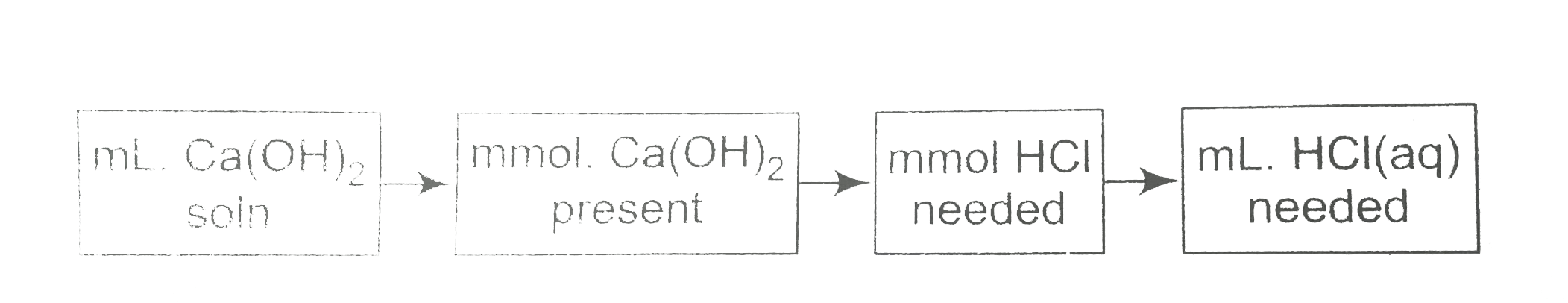
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-SOLUTIONS-QUESTION BANK (Building the knowledge)
- An ideal solution is formed when its components same
Text Solution
|
- If 100 mL of 1.00 M HCl and 100mL of 0.80 M NaOH solution are mixed, t...
Text Solution
|
- What volume of 0.00300 M Hcl solution would just neutralize 30.0 mL of...
Text Solution
|
- If the density of methanol is 0.793 kg L^(-1) what ia its volume neede...
Text Solution
|
- A sample of drinking water was found to be severely contaminated with ...
Text Solution
|
- H(2)Sgas is used in qualitative analysis of inorganic cations. Its sol...
Text Solution
|
- If m= molality of solution (mol Kg^(-1)) chi(solute)= mole fractio...
Text Solution
|
- Assuming ideal behavior, what mass of naphthalence (C(10)H(8)) would h...
Text Solution
|
- The vapour pressure of pure benzene at 88^(@)C is 957 mm and that of ...
Text Solution
|
- Vapor pressure of A ("molar mass" =78 mol^(-1)) and B ("molar mass" =9...
Text Solution
|
- Two liquids X and Y from an ideal solution at300K, Vapour pressure of ...
Text Solution
|
- The density ("in" g mL^(-1)) of a 3.60 M sulphuric acid solution that ...
Text Solution
|
- The density of 3M solution of NaCl is 1.25 g mL^(-1). The molality of ...
Text Solution
|
- In a 0.2 molal aqueous solution of weak acid HX (the degree of dissoci...
Text Solution
|
- A 5% solution of cane sugar is isotonic with 0.877% solution of urea. ...
Text Solution
|
- In a pair of immiscible liquida, a common solute dissolves in both and...
Text Solution
|
- If the equivalent mass of a metal is doble that of oxygen then the wei...
Text Solution
|
- Consider a solution of ethanol in water in which the mole fraction of ...
Text Solution
|
- The relation between molarity (C ) and molality (m) is given by )d= de...
Text Solution
|
- If C = molar concentration (mol L^(-1)) d = density of solution (Kg ...
Text Solution
|