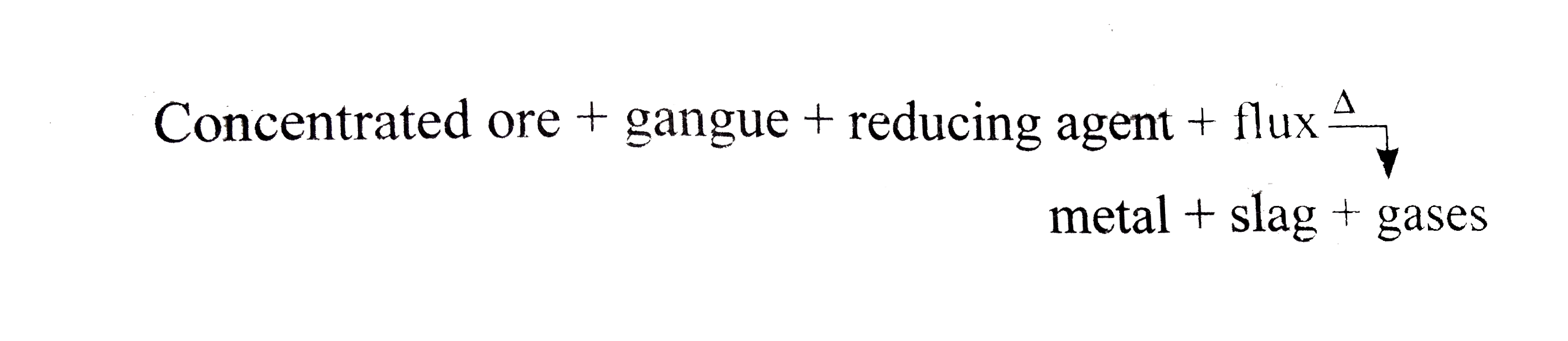A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPALS AND ISOLATION OF ELEMENTS
R SHARMA|Exercise Flow up (Test - 4)|18 VideosGENERAL PRINCIPALS AND ISOLATION OF ELEMENTS
R SHARMA|Exercise Flow up (Test - 5)|6 VideosGENERAL PRINCIPALS AND ISOLATION OF ELEMENTS
R SHARMA|Exercise Flow up (Test - 2)|6 VideosELECTROCHEMISTRY
R SHARMA|Exercise Follow-up Test 11|7 VideosNEET PREVIOUS PAPER
R SHARMA|Exercise Solved Question|51 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL PRINCIPALS AND ISOLATION OF ELEMENTS-Flow up (Test - 3)
- Calcination of ores involves heating the ore below its fusion tempera...
Text Solution
|
- Roasting of ores is done in
Text Solution
|
- By which of the following processes is an oxide ore readuced to the f...
Text Solution
|
- A flux is often added to remove impurities from a concentrated ore. ...
Text Solution
|
- Extraction of copper is done using copper pyrites. After roasting, th...
Text Solution
|
- which of the following metals can's be extracted by the the carbon red...
Text Solution
|
- Which of the following represents the thermite reaction ?
Text Solution
|