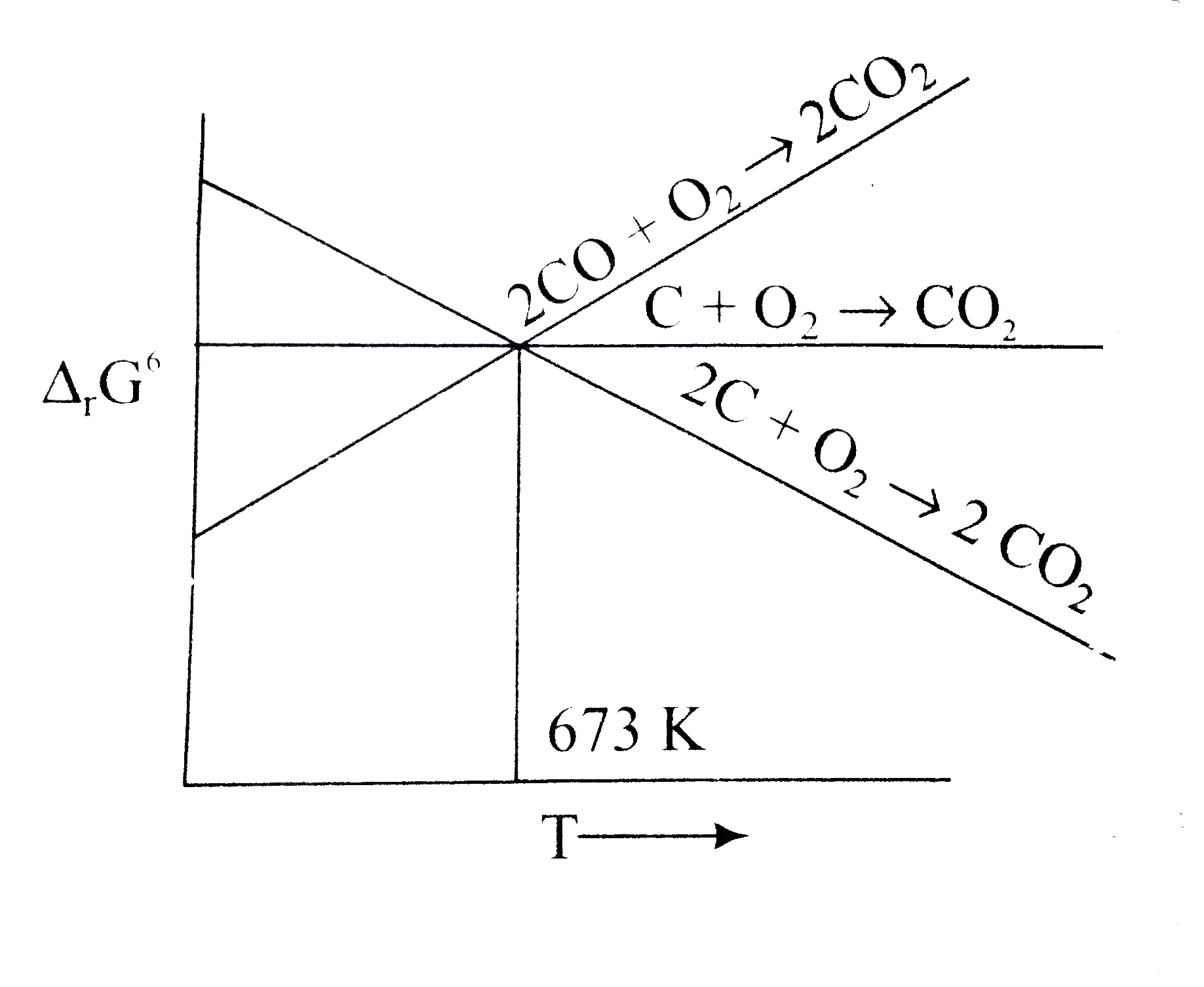
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
GENERAL PRINCIPALS AND ISOLATION OF ELEMENTS
R SHARMA|Exercise Flow up (Test - 5)|6 VideosGENERAL PRINCIPALS AND ISOLATION OF ELEMENTS
R SHARMA|Exercise Question bank|33 VideosGENERAL PRINCIPALS AND ISOLATION OF ELEMENTS
R SHARMA|Exercise Flow up (Test - 3)|7 VideosELECTROCHEMISTRY
R SHARMA|Exercise Follow-up Test 11|7 VideosNEET PREVIOUS PAPER
R SHARMA|Exercise Solved Question|51 Videos
Similar Questions
Explore conceptually related problems
R SHARMA-GENERAL PRINCIPALS AND ISOLATION OF ELEMENTS-Flow up (Test - 4)
- Ellngham diagram are plotwe of ……… as a function of temprecture
Text Solution
|
- Which of the following observations made from the Ellingham diagram is...
Text Solution
|
- Consider the following reaction at 1000^(@)C (A) Zn (s) + (1)/(2) O...
Text Solution
|
- DeltaG^(Ө) vs T plot in Ellingham diagram slopes downward for the reac...
Text Solution
|
- Which of the following faction is of no significance for roasting sul...
Text Solution
|
- Although thermodynamically feasible , in practices, magnesium metal a...
Text Solution
|
- The reduction of a metal oxide if the metals formed is in state at t...
Text Solution
|
- Which of the following statement is not correct?
Text Solution
|
- The most aendent are of iron is
Text Solution
|
- Iron is extracted on a commerical scale from Fe(2)O(3) by reduction wi...
Text Solution
|
- ( c) iron is the purest form of iron.
Text Solution
|
- When hard steel is heated to bright rednce reduce and then allowed to...
Text Solution
|
- Which of the following is malachile?
Text Solution
|
- Which of the following reaction is involved in the extraction of coppe...
Text Solution
|
- (xii) Which is known as 'blister copper' ?
Text Solution
|
- The flex in the smelting of copper is
Text Solution
|
- The main ore of zine is
Text Solution
|
- Zine is used in
Text Solution
|