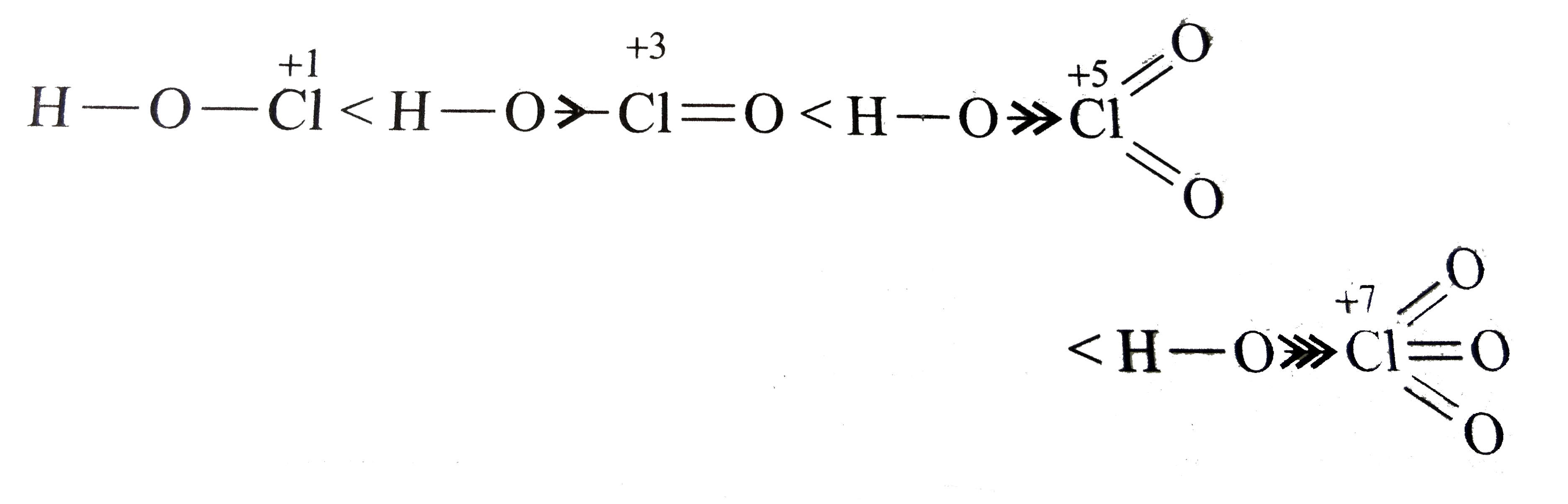A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE P BLOCK ELEMENTS-Follow-up Test 18
- Which of the following arrangements gives the correct order of increas...
Text Solution
|
- The ions ClO^(-), ClO(2)^(-), ClO(3)^(-) and ClO(4)^(-) are stabilized...
Text Solution
|
- Which of the following is most thermally stable?
Text Solution
|
- Which of the following is not the correct order of thermal stability?
Text Solution
|
- Which of the following compounds is used in the industry for bleaching...
Text Solution
|
- In hot solution (80^(@)), the sodium hypochlorite disproportionates ra...
Text Solution
|
- Which of the following reactions describes the decomposition of potass...
Text Solution
|
- The halogens reacts with each other to form interhalgon compounds.Thes...
Text Solution
|
- which of the following interhalogen does not exist?
Text Solution
|
- Which of the following interhalgon is not correctly matched with the ...
Text Solution
|
- Iodine reaxdily dissolves in KI solution due to
Text Solution
|