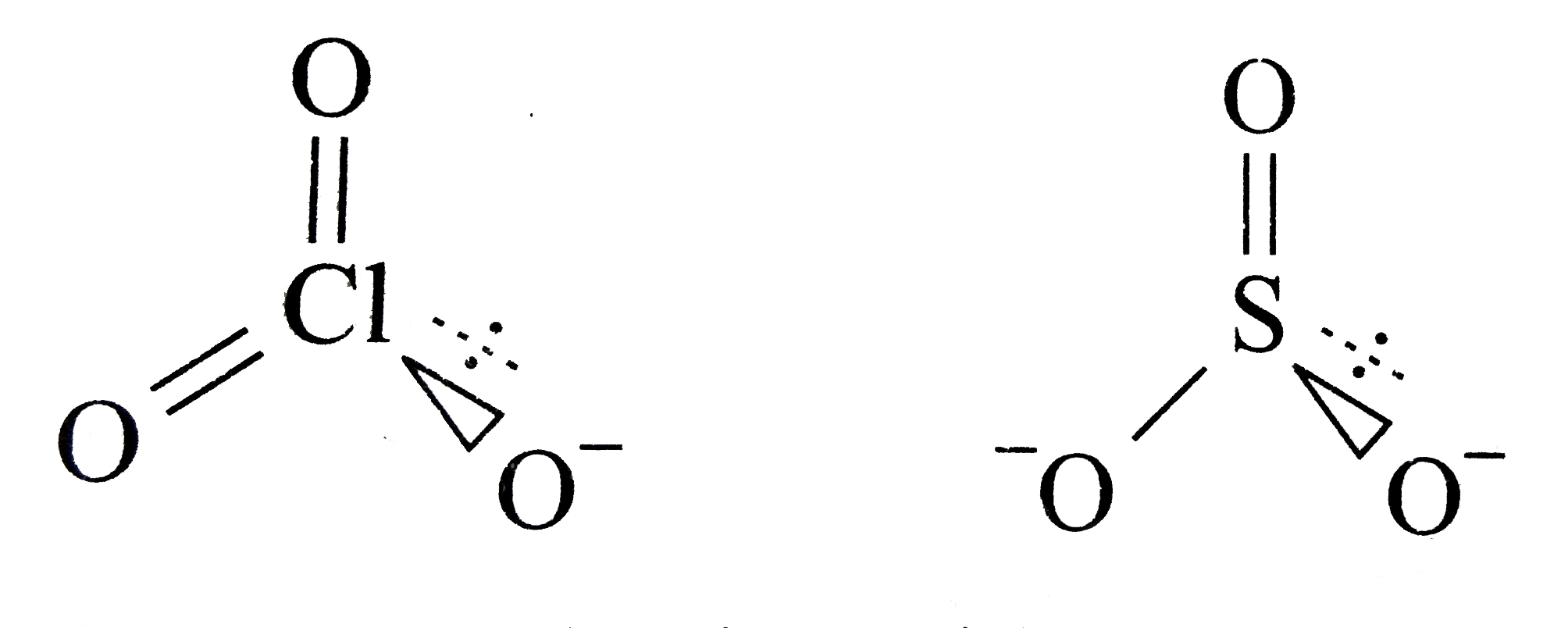
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE P BLOCK ELEMENTS-Archieves
- The variation of the boiling point of the hydrogen halides is in the o...
Text Solution
|
- Nitrogen dioxide and sulphur dioxide have some properties in common, w...
Text Solution
|
- Which of the following pairs of ions are isoelectronic and isostructur...
Text Solution
|
- Maximum bond angle at nitrogen is present in which of the following ?
Text Solution
|
- Which of the following species has plane tringular shape ?
Text Solution
|
- Acidity of diprotic acids in aqueous solutions increases in the order
Text Solution
|
- Indentify the correct order of solubility in aqueous medium
Text Solution
|
- Which is the strongest acid in the following ?
Text Solution
|
- Which of the following does not give oxygen on heating ?
Text Solution
|
- XeF(2) is isostructure with
Text Solution
|
- When CI(2) gas reacts with hot and concentrated sodium hydroxide solut...
Text Solution
|
- In which of the following compounds, nitrogen exhibits highest oxidati...
Text Solution
|
- A mixture of potassium chlorate ,oxalic acid and sulphuric acid is hea...
Text Solution
|
- Which of the following statement is not valid for oxoaids of phosphoru...
Text Solution
|
- Sulphur trioxide can be obtained by which of the following reactions:
Text Solution
|
- Which of the two lons from the list given have the geometry that is ex...
Text Solution
|
- Which one is the active constituent of bleaching powder?
Text Solution
|
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- The correct order of increasing bond angle in the following species is
Text Solution
|
- Oxidation state of P in H(4)P(2)O(5), H(4)P(2)O(6), H(4)P(2)O(7) are r...
Text Solution
|