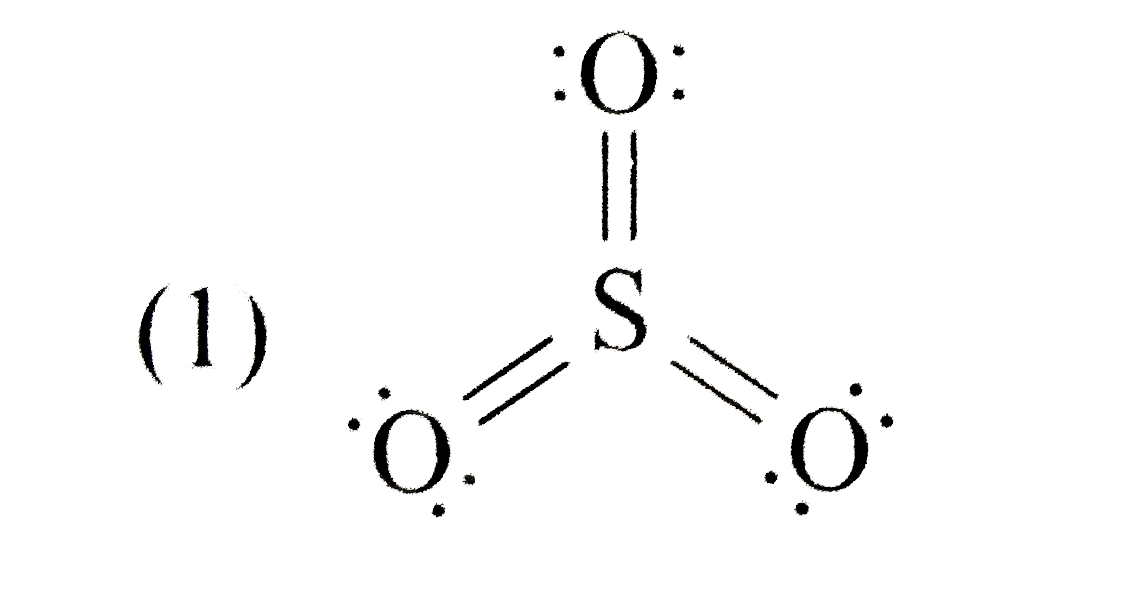
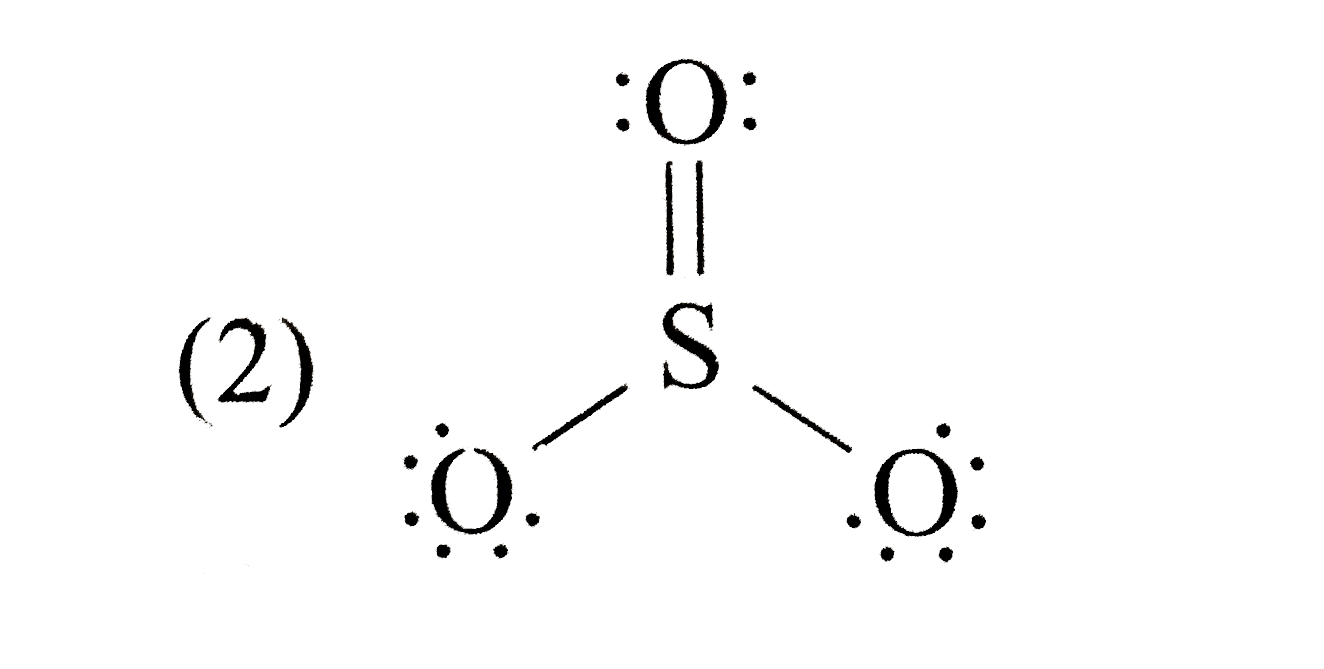
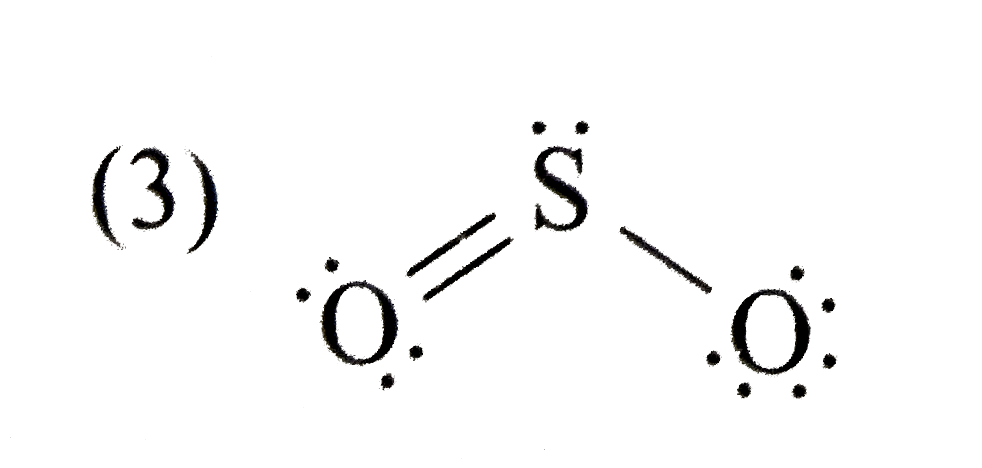
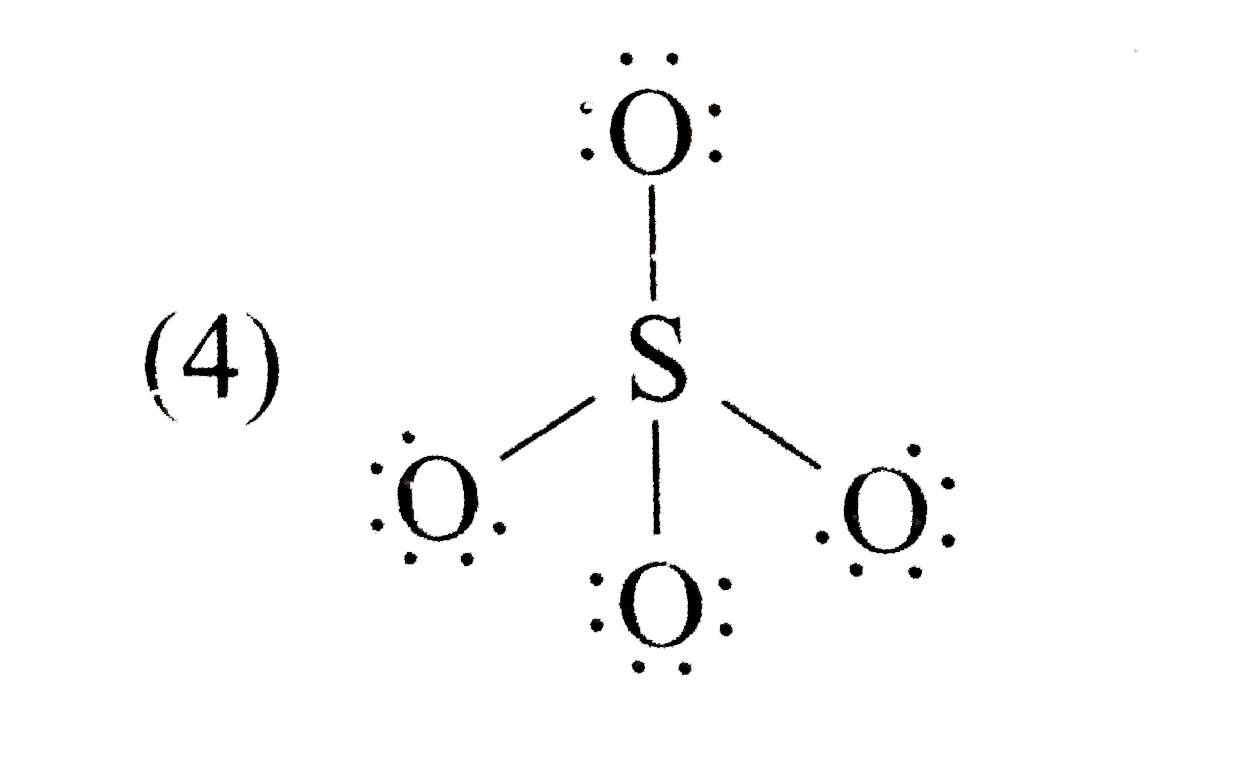
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
R SHARMA-THE P BLOCK ELEMENTS-Archieves
- Which of the two lons from the list given have the geometry that is ex...
Text Solution
|
- Which one is the active constituent of bleaching powder?
Text Solution
|
- Which of the following is a the most preferred and hence of the lower ...
Text Solution
|
- The correct order of increasing bond angle in the following species is
Text Solution
|
- Oxidation state of P in H(4)P(2)O(5), H(4)P(2)O(6), H(4)P(2)O(7) are r...
Text Solution
|
- Which one of the following arrangements represents the correct order o...
Text Solution
|
- In which one of the following species , the central atom has the tuype...
Text Solution
|
- How many bridging oxygen atoms are presents in P(4)O(10) ?
Text Solution
|
- Some of the properties of the two species, NO(3)^(-) and H(3)O^(+) are...
Text Solution
|
- In the case of alkali metals, the covalent character decreases in the ...
Text Solution
|
- Which of the following molecules acts as a Lewis acid?
Text Solution
|
- Which of the following is the strongest oxidising agent?
Text Solution
|
- In which of the following molecular/ions BF(2),NO(2)^(-),NH(2) and H(...
Text Solution
|
- Which one of the following arrangements does not give the correct pict...
Text Solution
|
- The angular shape of none molecule (O(3)) consists of
Text Solution
|
- The correct order for bond angles is :
Text Solution
|
- In which of the following pairs, the two spices are iso-structural?
Text Solution
|
- Which one of the following ionic species has the greatest proton affin...
Text Solution
|
- Which of the following species has a linear shape?
Text Solution
|
- Which of the following is not isostructural with SiCI(4) ?
Text Solution
|