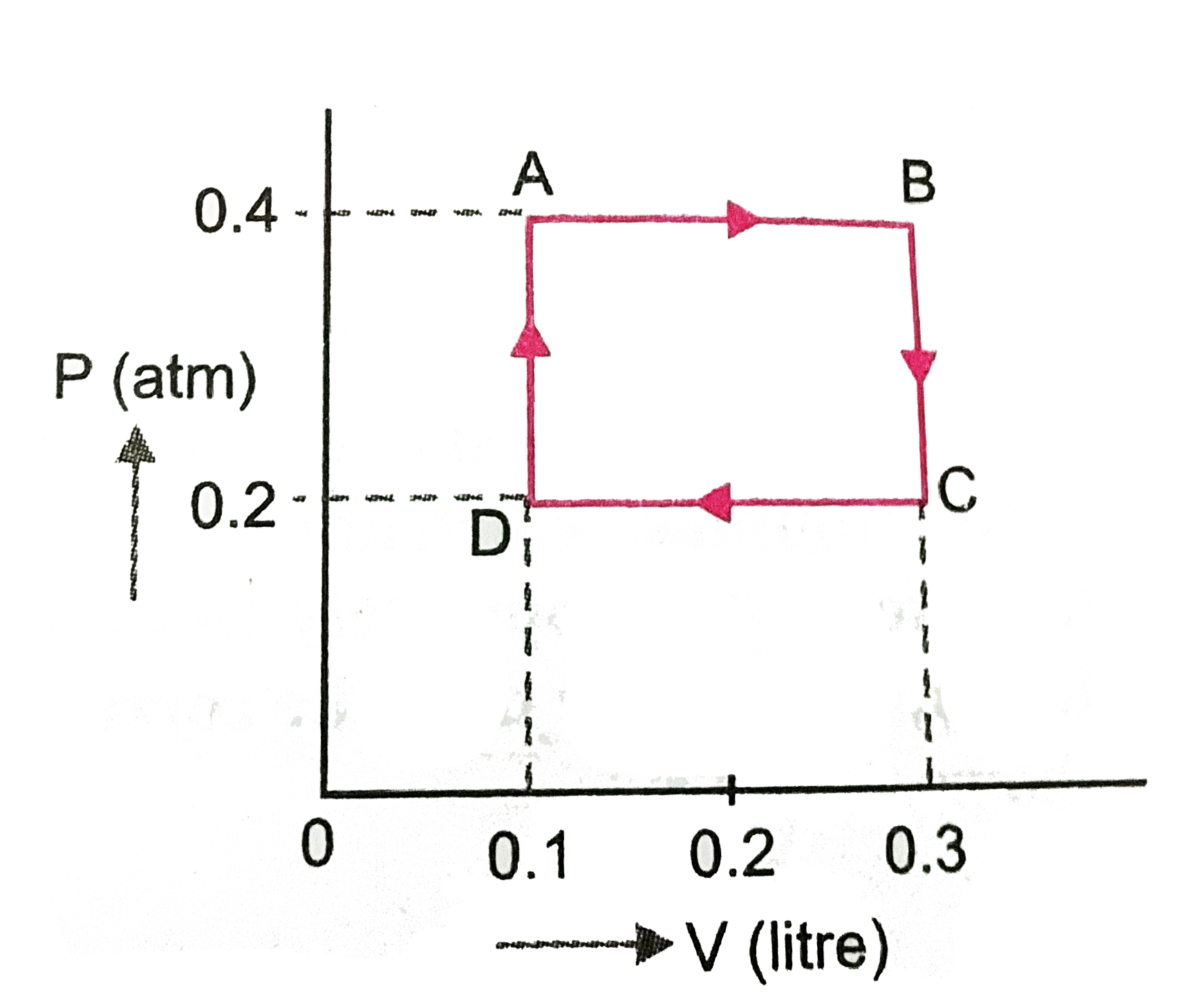Similar Questions
Explore conceptually related problems
Recommended Questions
- One mole of an ideal gas undergoes a cyclic change ABCDA as shown in (...
Text Solution
|
- A gas undergoes a cyclic process ABCDA as shown in the figure. The par...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic change ABCDA as shown in (...
Text Solution
|
- Two moles of a monoatomic ideal gas undergoes a process AB as shown in...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic change ABCD. From the give...
Text Solution
|
- An ideal gas undergoes cyclic process ABCDA as shown in givend p-V dia...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic process ABCDA as shown in ...
Text Solution
|
- एक मोल आदर्श गैस में चित्र में दर्शायें अनुसार चक्रीय परिवर्तन होता है...
Text Solution
|