Similar Questions
Explore conceptually related problems
Recommended Questions
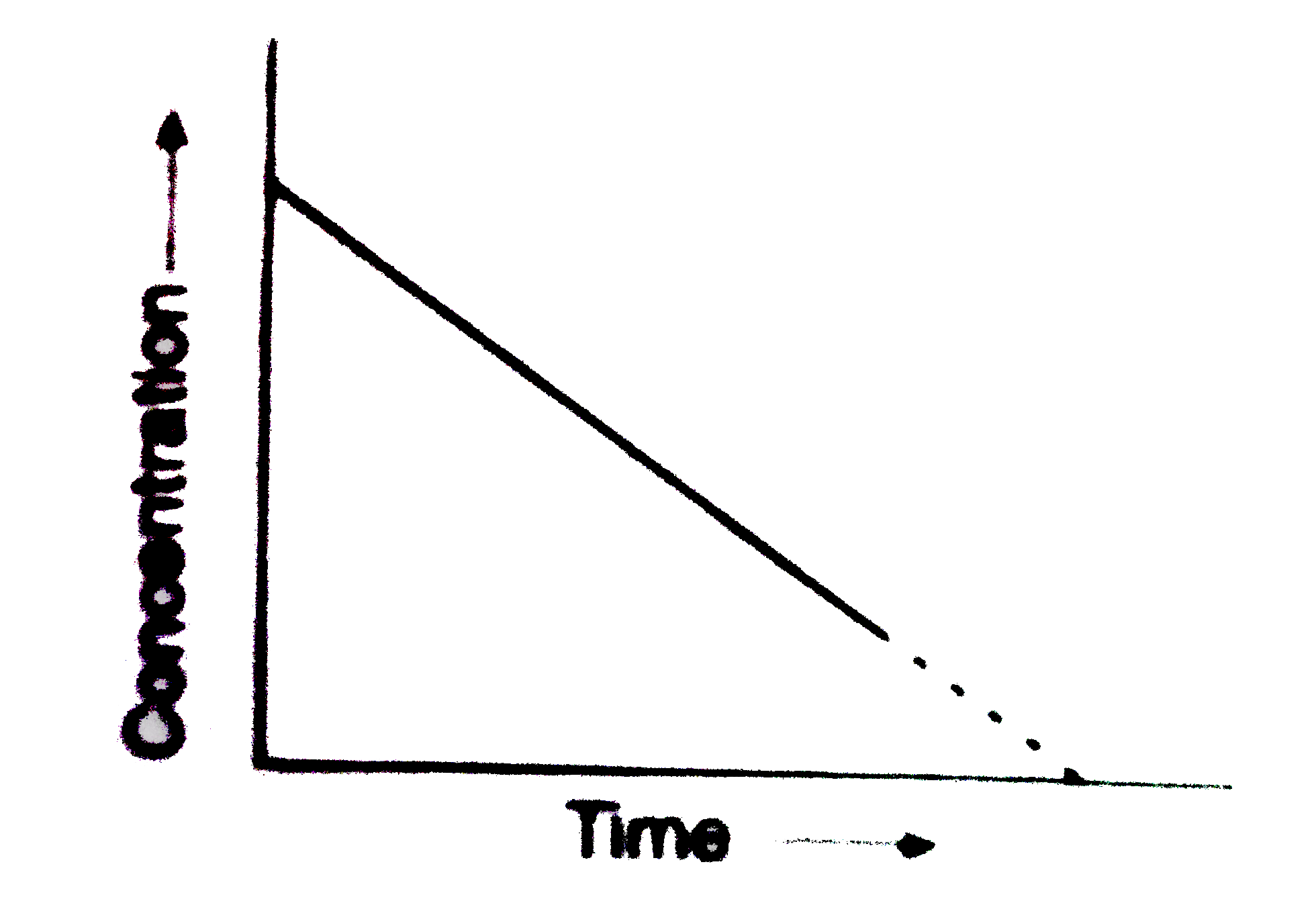
- For a zero order reaction the plot of concentration of reactant versus...
Text Solution
|
- For a reaction , a graph was plotted between reactant concentration c ...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- The plote between concentration versus time for a zero order reaction ...
Text Solution
|
- For a zero order reaction the plot of concentration of reactant versus...
Text Solution
|
- For a zero order reaction, the plot of concentration of a reactant vs ...
Text Solution
|
- The plote between concentration versus time for a zero order reaction ...
Text Solution
|
- In the plot of concentration of reactant versus time, the tangent at a...
Text Solution
|
- The plot between concentration versus time for a zero order reaction i...
Text Solution
|