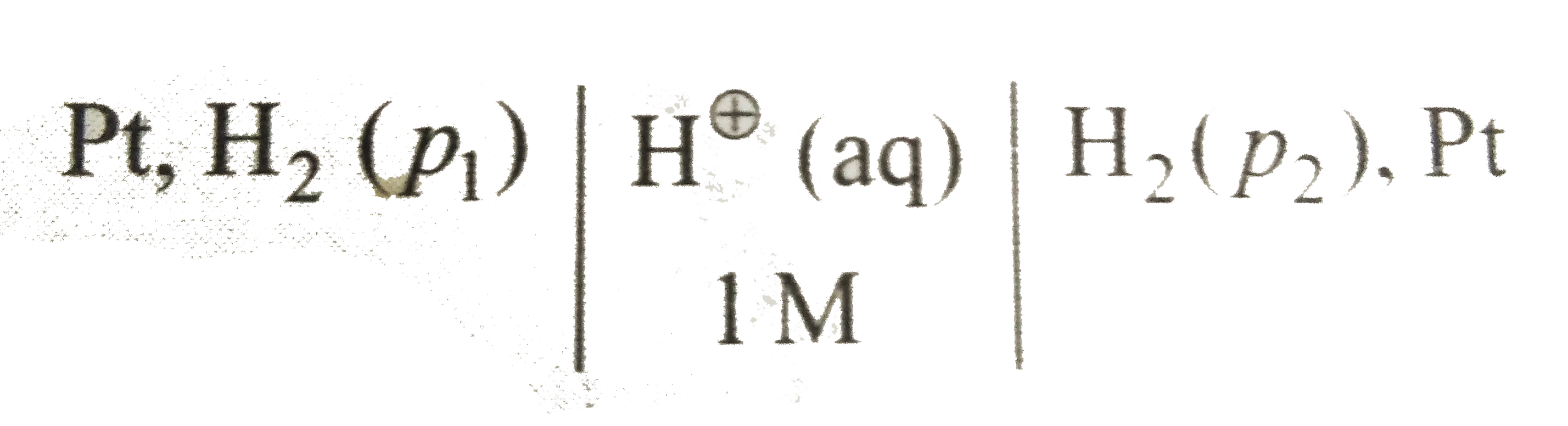Similar Questions
Explore conceptually related problems
Recommended Questions
- Consider the following cell with hydrogen electrodes at difference pre...
Text Solution
|
- Consider the following cell with hydrogen electrodes at difference pre...
Text Solution
|
- The emf of given cell Pt - underset(P(1))(H(2))|H^(+)|underset(P(2))(H...
Text Solution
|
- Galvanic cells generate electrical energy at the expense of a spontane...
Text Solution
|
- |underset("("p(1)")")(H(2))|underset("("p(1)")")(H^(+))|underset("("1M...
Text Solution
|
- What will be the emf for the given cell ? Pt|H(2)(g,P(1))|H^(+)(aq)|H(...
Text Solution
|
- Assuming that hydrogen begaves as an ideal gas, calculate EMF of the c...
Text Solution
|
- What will be the emf for the given cell ? Pt|H(2)(g,P(1))|H^(+)(aq)|...
Text Solution
|
- What will be the emf of given cell - Pt |H(2) (P(1)) | H((aq))^(+) | |...
Text Solution
|