Similar Questions
Explore conceptually related problems
Recommended Questions
- The dipole moment of HBr is 2.6 xx 10^(-30)Cm and interatomic spacing ...
Text Solution
|
- The dipole moment of HBr is 2.6 xx 10^(-30)Cm and interatomic spacing ...
Text Solution
|
- The H-O-H bond angle in the water molecule is 105^@ , the H -O bond di...
Text Solution
|
- The dipole moment of HBr is 1.6 xx 10^(-30) cm and interatomic spaci...
Text Solution
|
- HBr has dipole moment 2.6xx10^(-30)C-m. If the ionic character of the ...
Text Solution
|
- Calculate the fractional charge on each atom in HBr molecule. Given th...
Text Solution
|
- The H-O-H bond angle in the water molecule is 105^@ , theH -O bond dis...
Text Solution
|
- (a) A diatomic moleculae has a dipole moment of 1.2 D . If the bond di...
Text Solution
|
- Diatomic molecule has a dipole moment of 1.2D If its bond 1.0 Å what f...
Text Solution
|
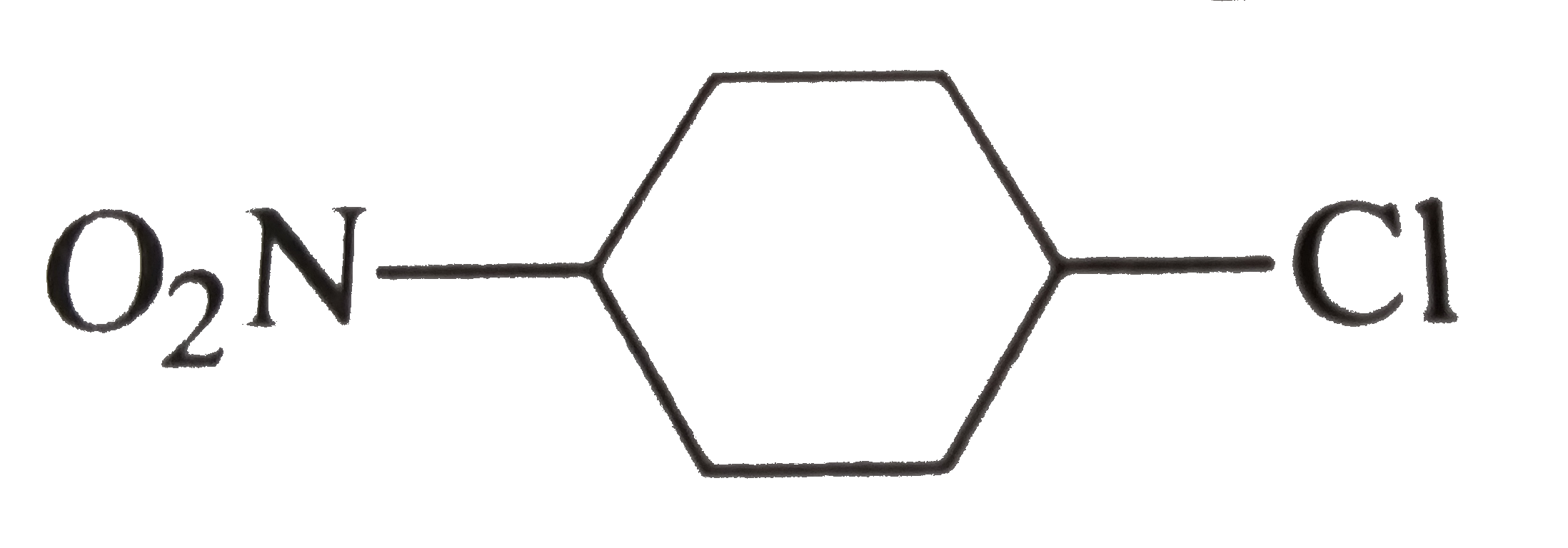 .
.