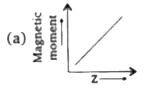
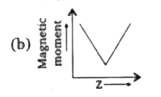
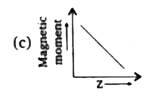
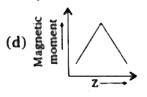
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
PERIODIC PROPERTIES
VK JAISWAL|Exercise Level 2|102 VideosPERIODIC PROPERTIES
VK JAISWAL|Exercise MATCH THE COLUMN|13 VideosPERIODIC PROPERTIES
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|22 Videosp-BLOCK ELEMENTS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|36 VideosQUALITATIVE INORGANIC ANALYSIS
VK JAISWAL|Exercise SUBJECTIVE PROBLEMS|5 Videos
Similar Questions
Explore conceptually related problems
VK JAISWAL-PERIODIC PROPERTIES-Level 1
- Which of the following metal is highest electropositive (metallic) in ...
Text Solution
|
- Which of the following species must have maximum number of electrons i...
Text Solution
|
- which of the following graph is correct representation between atomic ...
Text Solution
|
- If IUPAC name of an element is "unununium" then correct statement rega...
Text Solution
|
- Which property decreases from left to right across the periodic table ...
Text Solution
|
- Consider the following information about element P and Q: {:(,"Perio...
Text Solution
|
- which must represent an atom in an excited state?
Text Solution
|
- Which of the following anion has the smallest radius?
Text Solution
|
- The ionic radii of Li^(+), Be^(2+) and B^(3+) follow the order:
Text Solution
|
- Largest in size out of Na^(+),Ne and F^(-) is:
Text Solution
|
- Which of the following atom or ions has the smallest size?
Text Solution
|
- The single covalent radius of P is 0.11 nm. The single covalent radius...
Text Solution
|
- Which of the following is arranged in decreasing order of size?
Text Solution
|
- The correct order of increasing atomic radius of the following element...
Text Solution
|
- The correct order of increasing radii of the elementsSi,Al,Na and P is
Text Solution
|
- The size of the species, Pb,Pb^(2+), Pb^(4+) decreases as -
Text Solution
|
- Incorrect order of radius is:
Text Solution
|
- The correct order of atomic/ionic radiii is:
Text Solution
|
- The radius of which is closest to that of the Li^(+) ions?
Text Solution
|
- The first, second and third ionization energies (E(1),E(2)&E(3)) for a...
Text Solution
|