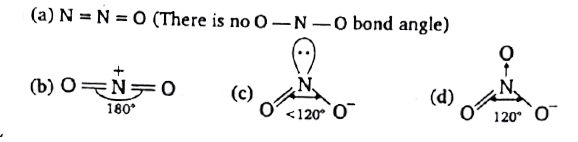
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- N -O -N bond angle is maximum in :
Text Solution
|
- N -O -N bond andle is maximum in :
Text Solution
|
- In which of the following O-N-O bond angles is highest ?
Text Solution
|
- Bond order of N-O bonds in nitrate ion is
Text Solution
|
- Why is the H-O-H bond angle in H(2)O smaller than H-N-H bond angle in ...
Text Solution
|
- The maximum number of N-Co-O bond angle in [Co(gly(3))]^(@) is :
Text Solution
|
- The maximum number of N-Co-O bond angle in [Co(gly(3))]^(@) is :
Text Solution
|
- The O-N-O bond angle in the nitrite ion, NO(2)^(-), is closest to :
Text Solution
|
- The bond order of the N-O bonds in NO3^- ion is
Text Solution
|