A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
VK JAISWAL-CHEMICAL BONDING (ADVANCED)-ONE OR MORE ANSWER IS/ARE CORRECT
- Which of the following statement is incorrect?
Text Solution
|
- Which of the following statements are not correct?
Text Solution
|
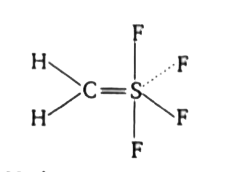
- In the structure of H(2)CSF(4), which of the following statement is/ar...
Text Solution
|
- In which compound compounds vacant hybride orbitals take part in bondi...
Text Solution
|
- Which of the following is true for N(2)O?
Text Solution
|
- Silane is more reactive than CH(4) towards Nu^(-) substitution due to...
Text Solution
|
- Which of the following statement(s) is/are not correct for following...
Text Solution
|
- If N(B) is the number of bonding electron and N(A) is the number of an...
Text Solution
|
- Stepwise hydrolysis of P(4)O(10) takes place via formation of :
Text Solution
|
- Select the correct statement(s) about the compound NO[BF(4)]:
Text Solution
|
- Which of the following molecule has as OO bonds?
Text Solution
|
- Which of the following species in paramagnetic:
Text Solution
|
- CO2 is not isostructural with
Text Solution
|
- Which of the following have a linear structure?
Text Solution
|
- Which of the following compound (s) is/are non-polar?
Text Solution
|
- Non-polar molecule are:
Text Solution
|
- Which of the following molecule species is/are having pi(2p) as H.O.M....
Text Solution
|
- Correct order of b.p.t is /are:
Text Solution
|
- Incorrect order between following compounds is/are:
Text Solution
|
- Which is correct statement ?
Text Solution
|