Similar Questions
Explore conceptually related problems
Recommended Questions
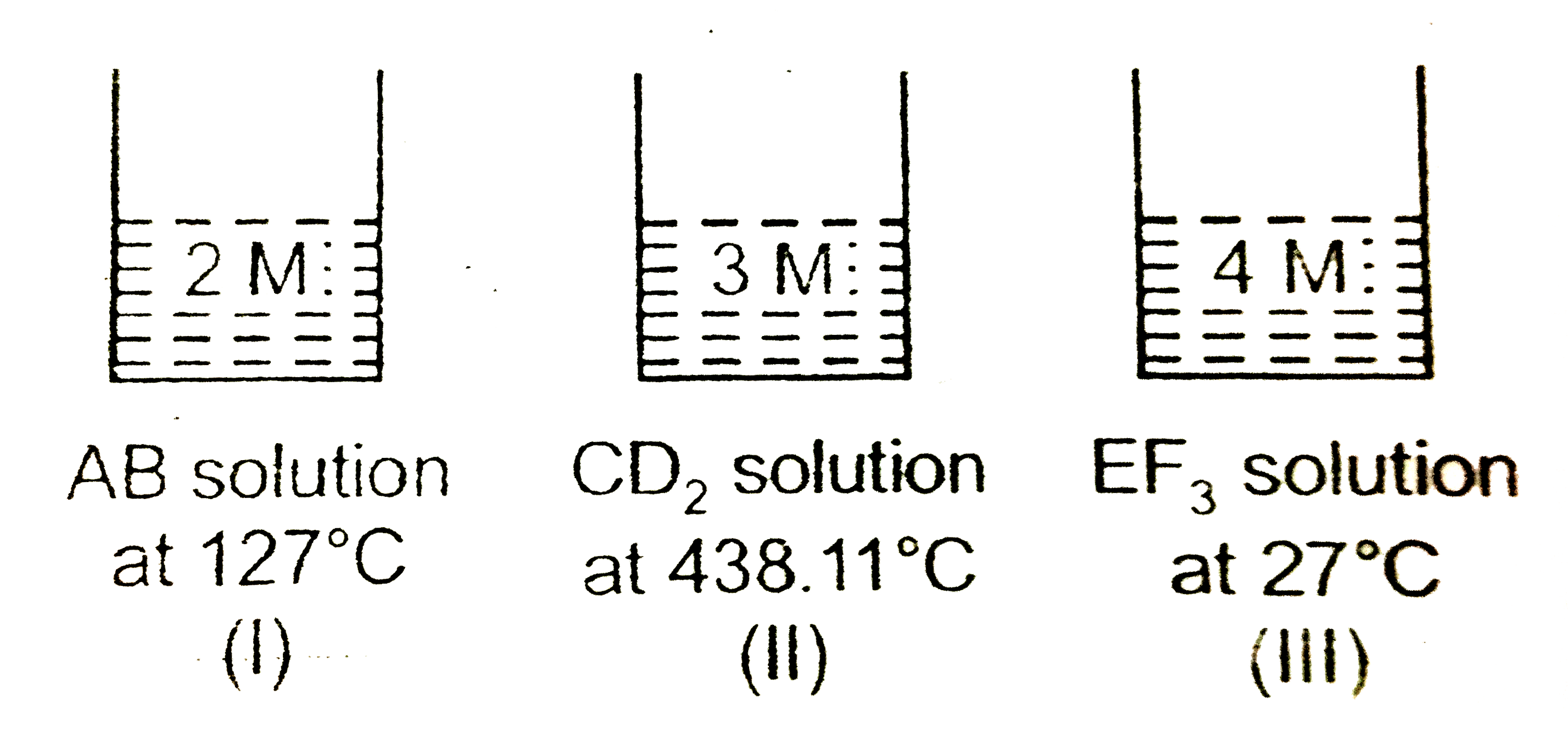
- Consider threee solutions of 3 strong electrolytes.AB, CD(2) and EF(3)...
Text Solution
|
- Arrange the following solutions in order of increasing osmotic pressur...
Text Solution
|
- The osmotic pressure of equimolar solution of urea, BaCI(2) and AICI(3...
Text Solution
|
- The osmotic pressure of equimolar solutions of urea, BaCl(2) and AlCl(...
Text Solution
|
- Consider threee solutions of 3 strong electrolytes.AB, CD(2) and EF(3)...
Text Solution
|
- निम्नलिखित में अन्तर स्पष्ट कीजिए । (i) विसरण एवं परासरण (ii ) प...
Text Solution
|
- If osmotic pressure of a solution is 2 atm at0^(@)C , then at 546K , ...
Text Solution
|
- Two equimolar solutions have osmotic pressures in the ratio of 2 : 1 a...
Text Solution
|
- Compare enol percent (I ) CH(3) - overset(O) overset(||) (C ) - CH...
Text Solution
|