Similar Questions
Explore conceptually related problems
Recommended Questions
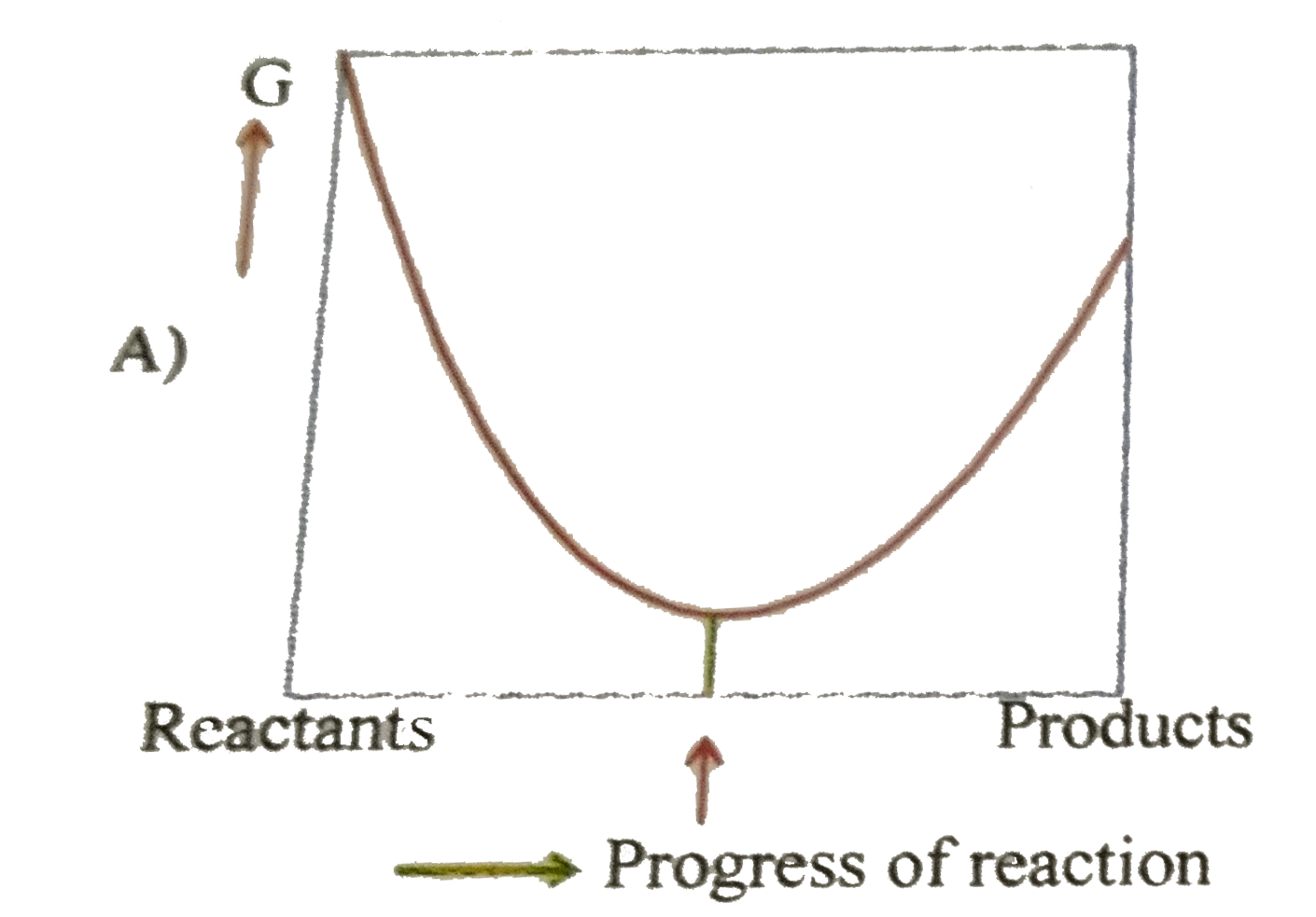
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- A schematic plot of log K(eq) vs inverse of temperature for a reaction...
Text Solution
|
- The equilibrium constant of a reaction and the standard Gibbs energy c...
Text Solution
|
- The magnitude of the equilibrium constant (K(eq)) for a cell reaction ...
Text Solution
|
- Kf and Kb are the velocity constants of forward and backward reactions...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- For a reversible reaction at constant temperature and at constant pres...
Text Solution
|
- The reaction which is in dynamic equilibrium, ensured us, that the rea...
Text Solution
|