Similar Questions
Explore conceptually related problems
Recommended Questions
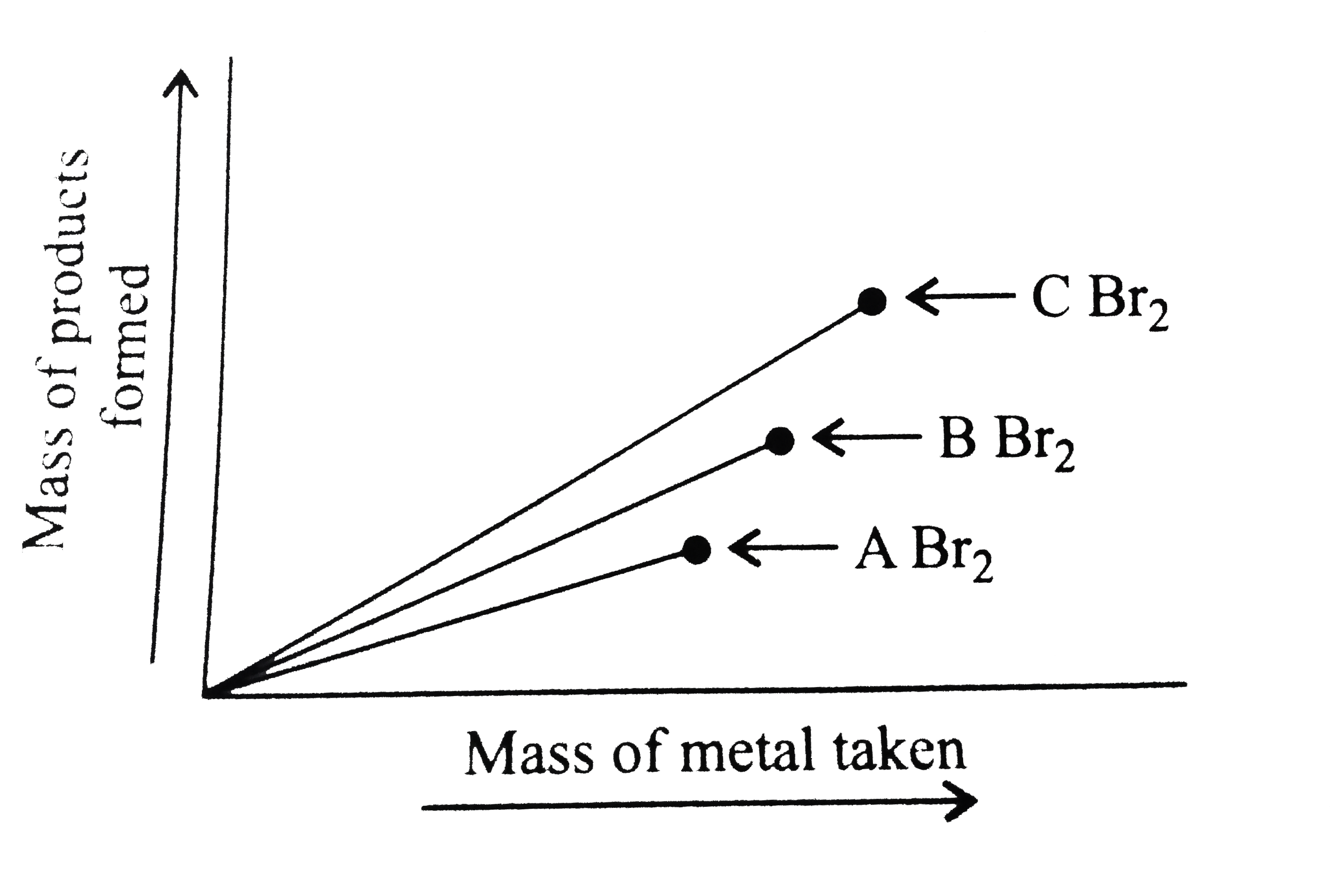
- Three metals of alkaline earth metal group (A,B and C) When reacted wi...
Text Solution
|
- Three metals of alkaline earth metal group (A,B and C) When reacted wi...
Text Solution
|
- The IE(1) and IE(2) (kJ mol^(-1)) of three elements A, B and C are giv...
Text Solution
|
- Compare the alkali metals and alkaline earth metals with respect to (a...
Text Solution
|
- Compare alkaline earth metals with alkali metals w.r.t (a) atomic and ...
Text Solution
|
- Three identical vessels are filled with equal masses of three differen...
Text Solution
|
- Two oxides of metals A and B are isomorphous . The metal A whose atomi...
Text Solution
|
- Consider a reaction A(g)overset(k=0.1 M min^(-1))to2B(g). If initial c...
Text Solution
|
- Alk aline earth metal (A), belongs to 3rd period reacts with oxygen an...
Text Solution
|