Similar Questions
Explore conceptually related problems
Recommended Questions
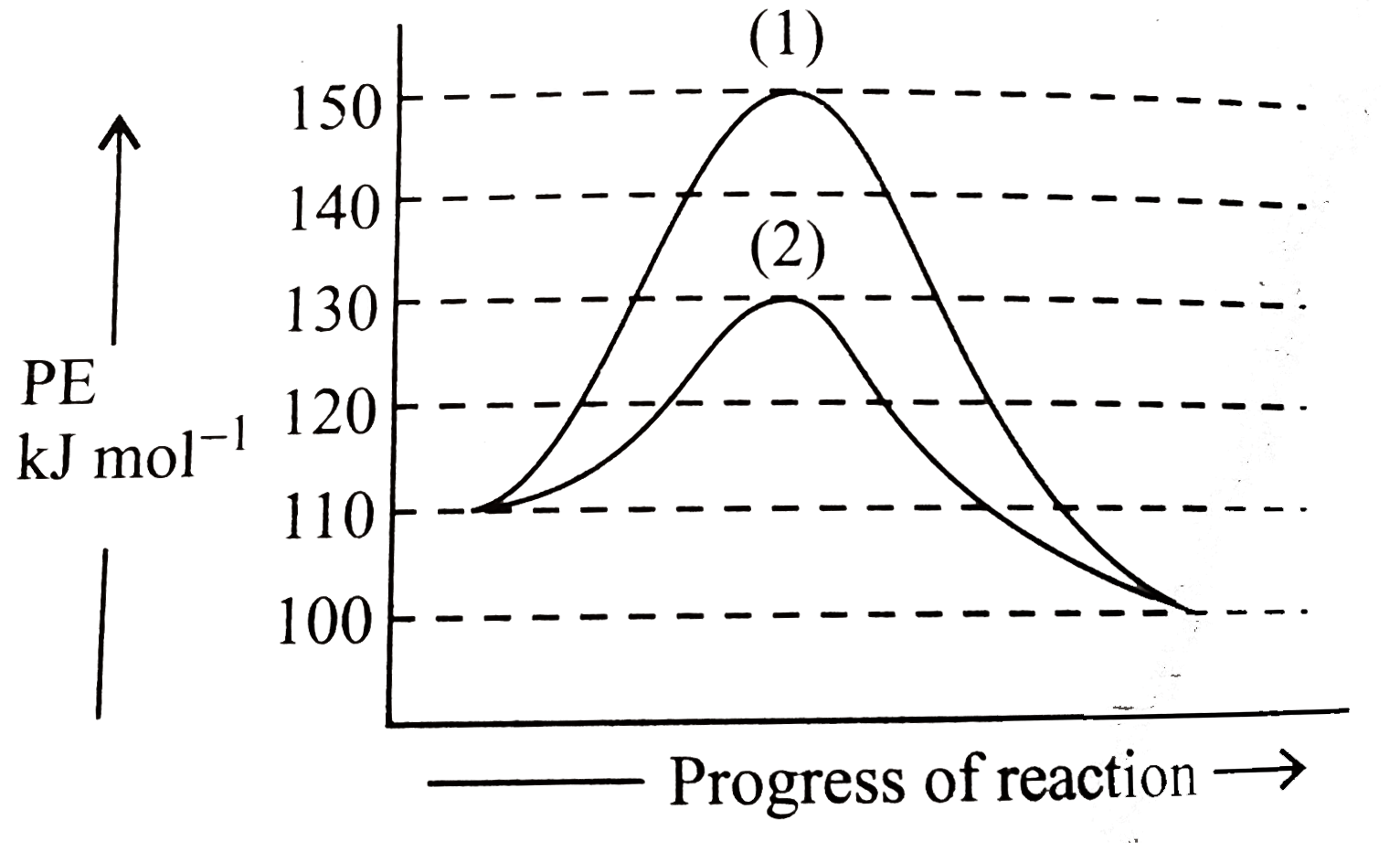
- Given the following graph. (a) Calculate Delta H for the reaction an...
Text Solution
|
- Given the following graph. (a) Calculate Delta H for the reaction an...
Text Solution
|
- Refer picture given below. (a) Calculate DeltaH for the reaction and...
Text Solution
|
- For a given reaction, presence of catalyst reduces the energy of activ...
Text Solution
|
- From the given figure: i) Calculate DeltaE for the reaction and ene...
Text Solution
|
- Assertion: A catalyst increases the rate of a reaction. Reason: In pre...
Text Solution
|
- For a reaction A(2)+B(2) hArr 2AB the figure shows the path of the rea...
Text Solution
|
- At 27^(@)C in the presence of a catalyst, the activation ?energy of a...
Text Solution
|
- In the presence of catalyst, activation energy for forward reaction………...
Text Solution
|