Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
CHEMICAL KINETICS
PRADEEP|Exercise CURIOSITY QUESTION|2 VideosCHEMICAL KINETICS
PRADEEP|Exercise TEST YOUR GRIP (MULTIPLE CHOICE QUESTIONS)|25 VideosCHEMICAL KINETICS
PRADEEP|Exercise ADVANCED PROBLEMS FOR COMPETITIONS|14 VideosBIOMOLECULES
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|25 VideosCHEMISTRY IN EVERYDAY LIFE
PRADEEP|Exercise IMPORTANT QUESTION FOR BOARD EXAMINATION|30 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL KINETICS-PROBLEMS FOR PRACTICE
- The decomposition of N(2)O(5) in a carbon tetrachloride solutioin has ...
Text Solution
|
- For the following reaction: 2A + B +C rarr A(2)B + C The rate la...
Text Solution
|
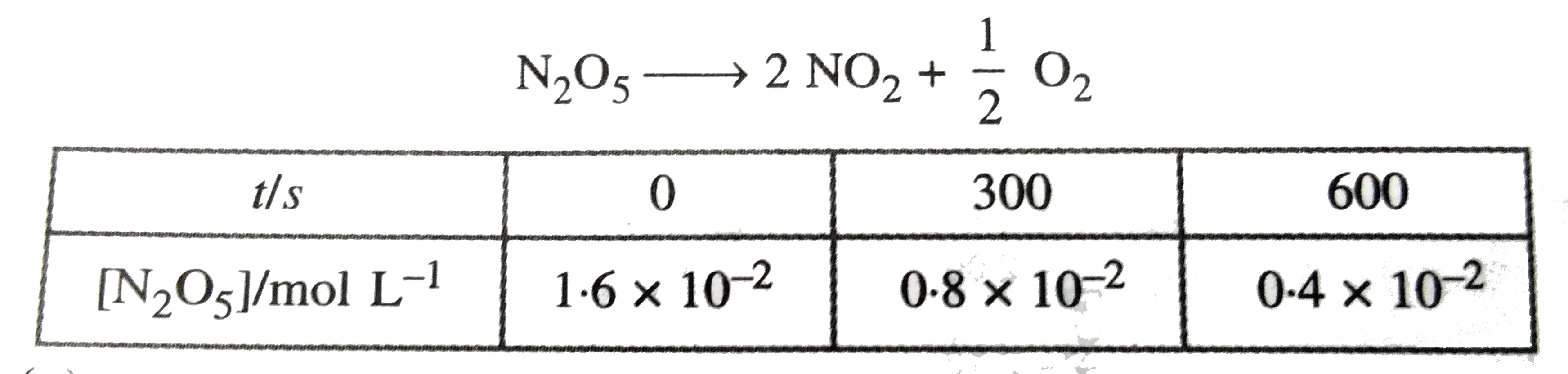
- Following data are obtained for the reaction N(2)O(5)to2 NO(2)+(1)/(...
Text Solution
|
- For a gaseous reaction 2A+B(2)to2AB, the following rate data were obta...
Text Solution
|
- For the reaction 2N(2)O(5)(g)to4NO(2)(g)+O(2)(g), the following result...
Text Solution
|
- For the thermal decomposition of acetaldehyde, CH(3)CHO(g)to CH(4)(g)+...
Text Solution
|
- The initial rate of reaction A+5B+6Cto3L+3M has been determined by mea...
Text Solution
|
- The following data were reported for the decompoistion of N(2)O(5) in ...
Text Solution
|
- The rate of decomposition of N(2)O(5) in C Cl(4) solution has been stu...
Text Solution
|
- The catalysed decomposition of H(2)O(2) in aqueous solution is followe...
Text Solution
|
- Methyl acetate was subjected to hydrolyiss in N-HCl at 298 K. 5mL of t...
Text Solution
|
- A 20% solution of cane sugar having dextrorotation of 34.50 inverted b...
Text Solution
|
- A first order reaction is found to have a rate constant k=7.39xx10^(-5...
Text Solution
|
- Time for half change for a first order reaction is 25 minutes. What ti...
Text Solution
|
- The half life period of a first order reaction is 60 minutes. What per...
Text Solution
|
- It was found that a solution of cane sugar was hydrolysed to the exten...
Text Solution
|
- Decomposition of a gas is of first order. It takes 80 minutes for 80% ...
Text Solution
|
- The data for the conversion of compound A into its isomeride B are as ...
Text Solution
|
- The following data were obtained during the catalysed decomposition of...
Text Solution
|
- Find the two-thirds life (t(2//3)) of a first order reaction in which ...
Text Solution
|