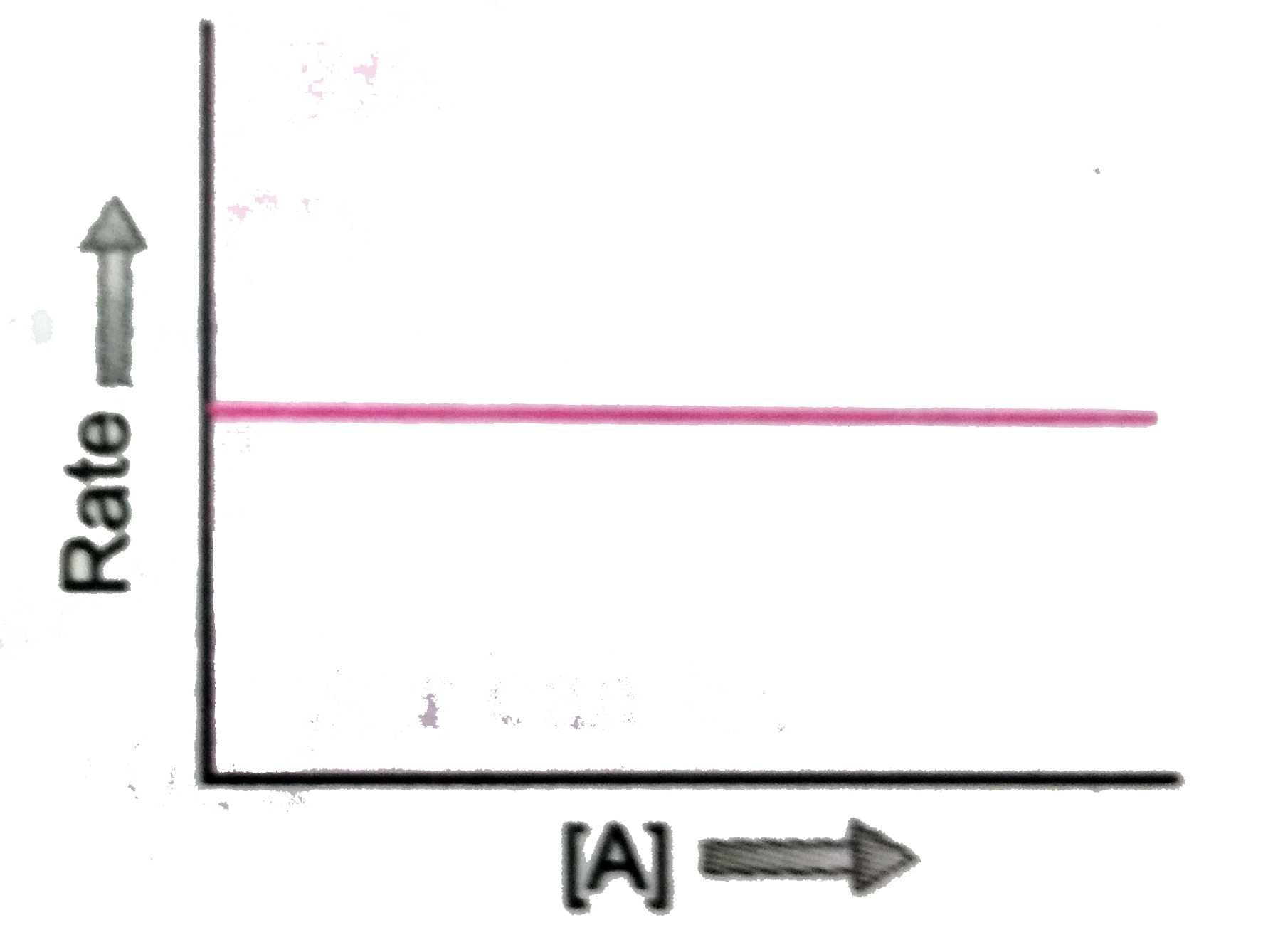
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
PRADEEP|Exercise CONCEPTUAL QUESTIONS (III. Molecularity of a reaction, its mechanism and difference between order and molecularity)|2 VideosCHEMICAL KINETICS
PRADEEP|Exercise CONCEPTUAL QUESTIONS (IV. Integrated rate equations, half-life, determination of rate law, rate constant and order)|6 VideosCHEMICAL KINETICS
PRADEEP|Exercise CONCEPTUAL QUESTIONS (I. General introduction and rates of reactions)|6 VideosBIOMOLECULES
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|25 VideosCHEMISTRY IN EVERYDAY LIFE
PRADEEP|Exercise IMPORTANT QUESTION FOR BOARD EXAMINATION|30 Videos
Similar Questions
Explore conceptually related problems