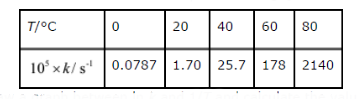
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL KINETICS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTTIONS (MULTIPLE CHOICE QUESTIONS-1)|20 VideosCHEMICAL KINETICS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTTIONS (MULTIPLE CHOICE QUESTIONS-II)|12 VideosCHEMICAL KINETICS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS & PROBLEMS)|9 VideosBIOMOLECULES
PRADEEP|Exercise IMPORTANT QUESTIONS (FOR BOARD EXAMINATION)|25 VideosCHEMISTRY IN EVERYDAY LIFE
PRADEEP|Exercise IMPORTANT QUESTION FOR BOARD EXAMINATION|30 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-CHEMICAL KINETICS-NCERT EXERCISES
- The following rate data were obtained at 303K for the following reacti...
Text Solution
|
- The reaction between A and B is first order with respect to A and zero...
Text Solution
|
- Calculate the half life of a first order reaction from their rate cons...
Text Solution
|
- The half-life for radioactive decay of ""^(14)C is 5730 y. An archael...
Text Solution
|
- The experimental data for the decomposition of N(2)O(5)[2N(2)O(5)to4NO...
Text Solution
|
- The rate constant for a first order reaction is 60s^(-1). How much tim...
Text Solution
|
- During nuclear explosion, one of the products is .^(90)Sr with half- l...
Text Solution
|
- For a first order reaction, show that the time required for 99% comple...
Text Solution
|
- A first order reaction takes 40 min for 30% decomposition. Calculate t...
Text Solution
|
- For the decomposition of azoisopropane to hexane and nitrogen at 54 ...
Text Solution
|
- The following data were obtained during the first order thermal dec...
Text Solution
|
- The rate constant for the decomposition of N(2)O(5) at various tempera...
Text Solution
|
- The rate constant for the decomposition of hydrocarbons is 2.418xx10^(...
Text Solution
|
- Consider a certain reaction A rarr Products with k=2.0xx10^(-2)s^(-1)....
Text Solution
|
- Sucrose decomposes in acid solution into glucose and fructose accordin...
Text Solution
|
- The decomposition of hydrocarbon follows the equation k=(4.5xx10^(11)...
Text Solution
|
- The rate constant for the first order decompoistion of a certain react...
Text Solution
|
- The decomposition of A into product has value of k as 4.5xx10^(3)s^(-...
Text Solution
|
- The time required for 10% completion of a first order reaction at 298K...
Text Solution
|
- The rate of a particular reaction doubles when temperature changes fr...
Text Solution
|