Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT SOLVED QUESTIONS)|9 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS)|9 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise TEST YOUR GRIP (FILL IN THE BLANKS)|21 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|27 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HALOALKANES AND HALOARENES-CONCEPTUAL QUESTIONS
- Predict the order of reactivity of the following compounds in S(N)1 re...
Text Solution
|
- Which reaction in each pair shown below will show the faster rate of d...
Text Solution
|
- The following reactions involve reactions of an alkyl bromide with two...
Text Solution
|
- The following reaction gives two products. C(6)H(5)CH(2)CHClC(6)H(5)...
Text Solution
|
- Write down the structure of the product of the following reaction. C...
Text Solution
|
- An alkyl halide (A), on reaction with magnesium in dry ether followed ...
Text Solution
|
- An alkyl halides (P) reacts with magnesium metal in presence of dry et...
Text Solution
|
- Write the major product of the following reaction: (a) CH(2)=CHBr un...
Text Solution
|
- Differentiate between chiral and achiral molecules.
Text Solution
|
- Identify and indicate the presence of centre of chirality, if any, in ...
Text Solution
|
- What is meant by chirality of a compound ? Give an example.
Text Solution
|
- What are enantiomers? Draw the structures of the possible enantiomers ...
Text Solution
|
- Distinguish between enantiomers and diastereomers.
Text Solution
|
- Differentiate between retention and inversion.
Text Solution
|
- (+-)-2-Butanol is optically inactive. Give reasons.
Text Solution
|
- Optically active 2-iodo butane on treatment with NaI in acetone gives ...
Text Solution
|
- (i) Which alkyl halide from the followng pair is chiral and undergoes ...
Text Solution
|
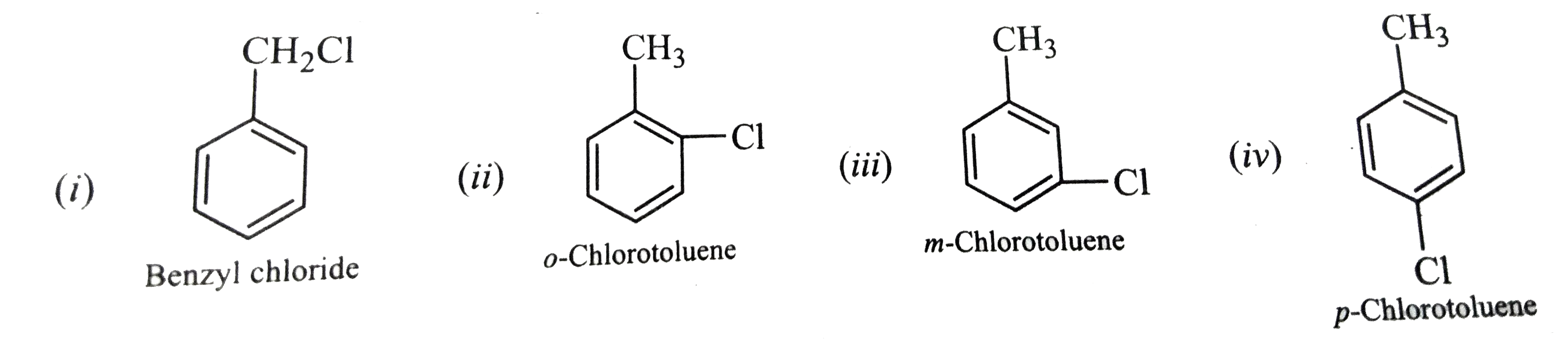
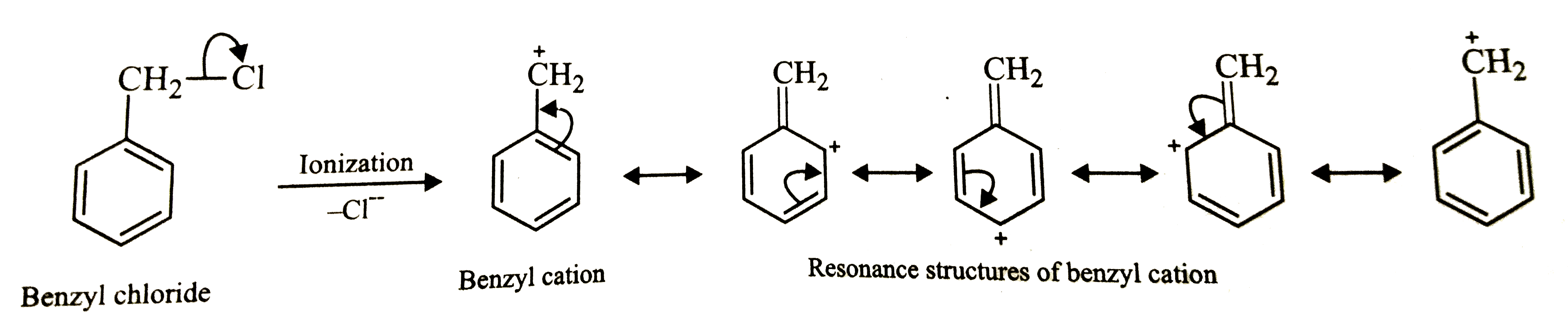
- Out of the various possible isomers of C7H7Cl containing a benzene rin...
Text Solution
|
- p-Chloronitrobenzene undergoes nulceophilic substitution faster than c...
Text Solution
|
- (i) Complete the following, giving the structures of the pincipal orga...
Text Solution
|