Text Solution
Verified by Experts
Topper's Solved these Questions
HALOALKANES AND HALOARENES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MATCHING TYPE QUESTIONS)|15 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (Assertion and reason type questions)|1 VideosHALOALKANES AND HALOARENES
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (MULTIPLE CHOICE QUESTIONS-II)|11 VideosGENERAL PRINCIPLES AND PROCESSES OF ISOLATION OF ELEMENTS
PRADEEP|Exercise Curiosity Questions|2 VideosORGANIC COMPOUNDS CONTAINING NITROGEN
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|27 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-HALOALKANES AND HALOARENES-NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTIONS (SHORT ANSWER QUESTIONS)
- Haloarenas are less reactive than haloalkanes and haloalkenes. Explain...
Text Solution
|
- Discuss the role of Lewiis acids in the preparation of aryl bromides a...
Text Solution
|
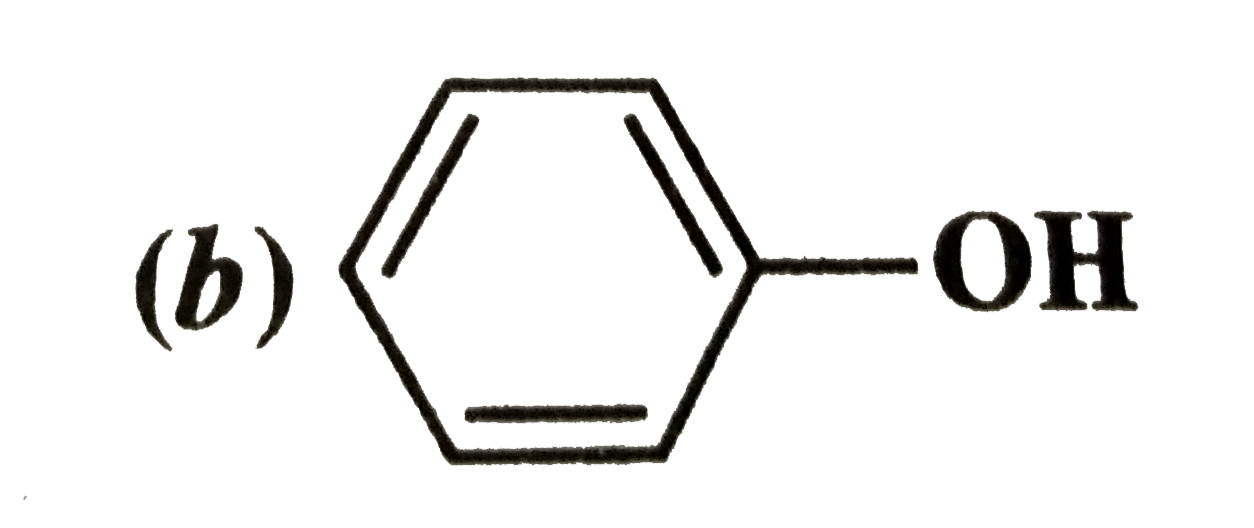
- Which of the following compounds (a) and (b) will not react with a mix...
Text Solution
|
- Which of the products will be major product in the reaction given belo...
Text Solution
|
- Why is the solubility of haloalkanes in water very low ?
Text Solution
|
- Draw other resonance structures related to the following structure and...
Text Solution
|
- Classify the following compounds as primary, secondary and tertiary ha...
Text Solution
|
- Compound 'A' with molecular formula C(4)H(9)Br is treated with aq. KOH...
Text Solution
|
- Write the structures and names of the compounds formed when compound '...
Text Solution
|
- Identify the product A and B formed in the following reaction: CH(3)...
Text Solution
|
- Which of the following compounds will have the highest melting point a...
Text Solution
|
- Write down the structure and IUPAC name for neo-pentylbromide.
Text Solution
|
- A hydrocarbon of molecular mass 72 g mol^(-1) gives a single monochlor...
Text Solution
|
- Name of the alkene which will yield 1-chloro-1-methylcyclohexane by it...
Text Solution
|
- Which of the following haloalkanes reacts with aqueous KOH most easily...
Text Solution
|
- Why can aryl halides not be prepared by reaction of phenol with HCl in...
Text Solution
|
- Which of the following compounds would undergo S(N)1 reaction faster a...
Text Solution
|
- Allyl chloride is hydrolysed more readily than n-propyl chloride. Why ...
Text Solution
|
- Why is it necessary to avoid even traces of moisture during the use of...
Text Solution
|
- How do polar solvents help in the first step in S(N)1 mechanism?
Text Solution
|