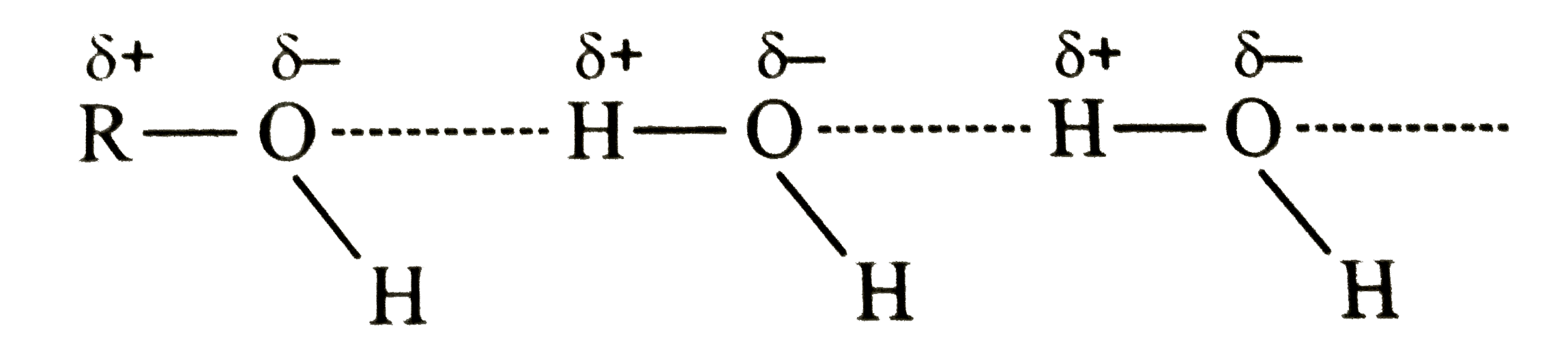Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (MULTIPLE CHOICE QUESTIONS-I)|16 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (MULTIPLE CHOICE QUESTIONS-II)|5 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS)|12 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALCOHOLS, PHENOLS AND ETHERS-NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT EXERCISES)
- i. Draw the structures of all isomeric alcohols of molecular formula ...
Text Solution
|
- Explain why propanol has a higher boiling point than hydrocarbon butan...
Text Solution
|
- Alcohols are comparatively more soluble in water than hydrocarbons of ...
Text Solution
|
- What is meant by hydroboration-oxidation reaction ? Illustrate it with...
Text Solution
|
- Give the structures and IUPAC names of monohydric phenols of molecular...
Text Solution
|
- While separating a mixture of ortho- and para-nitrophenols steam disti...
Text Solution
|
- Give the equations of reaction for the preparation of phenol form cume...
Text Solution
|
- Write the chemical reaction for the preparation of phenol form chlorob...
Text Solution
|
- Write the mechanism of hydration of ethene to yield ethanol.
Text Solution
|
- You are given benzene, conc. H(2)SO(4), and NaOH. Write the equations ...
Text Solution
|
- Show how will you synthesie: i. 1-Phenylethanol form a suitable alk...
Text Solution
|
- Give two reactions that show the acidic nature of phenol. Compare the ...
Text Solution
|
- Explain why is ortho-nitrophenol more acidic than ortho-methoxyphenol ...
Text Solution
|
- Explain how does the (---OH) group attached to a carbon of benzene rin...
Text Solution
|
- Give the equations of the following reactions: i. Oxidation of prop...
Text Solution
|
- Explain the following with an example: i. Kolbe's reaction ii. R...
Text Solution
|
- Write the mechanism of acid dehydration of ethanol to yield ethene.
Text Solution
|
- How are the following conversions carried out ? i. Propene rarr Pro...
Text Solution
|
- Name the reagents used in the following reactions: i. Oxidation of ...
Text Solution
|
- Given reason for the higher boiling point of ethanol in comparison to ...
Text Solution
|