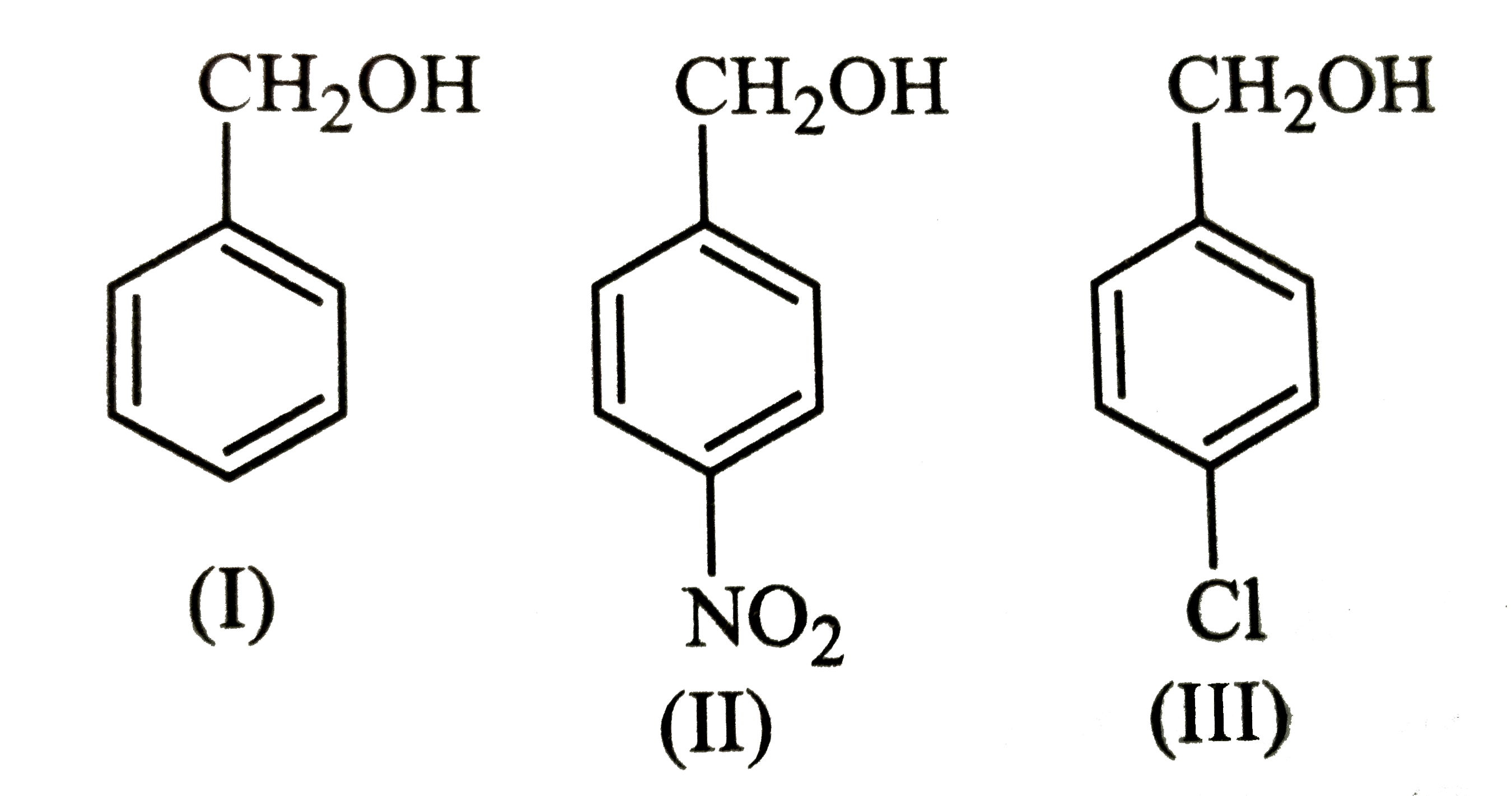
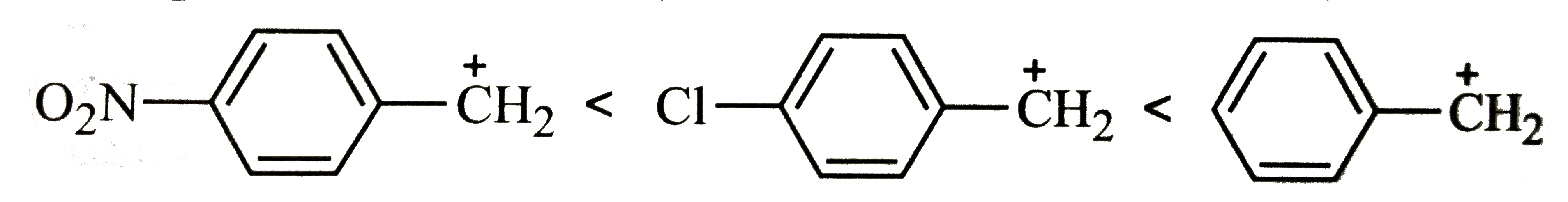
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (MULTIPLE CHOICE QUESTIONS-II)|5 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (SHORT ANSWER QUESTIONS)|35 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT EXERCISES)|33 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALCOHOLS, PHENOLS AND ETHERS-NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (MULTIPLE CHOICE QUESTIONS-I)
- Monochlorination of toluene in sunlight followed by hydroysis with aq....
Text Solution
|
- How many alcohols with molecular formula C(4)H(10)O are chiral in natu...
Text Solution
|
- What is the correct order of reactivity of alcohols in the following r...
Text Solution
|
- CH(3)CH(2)OH can be converted into CH(3)CHO by........... .
Text Solution
|
- The process of converting alkyl halides into alcohols involves...........
Text Solution
|
- Which of the following compounds is aromatic alcohol?
Text Solution
|
- Give IUPAC name of the compound given below. CH(3)-underset(Cl)under...
Text Solution
|
- IUPAC name of m-cresol is.......... .
Text Solution
|
- IUPAC name of m-cresol is.......... .
Text Solution
|
- Which of the following species can act as the strongest base ?
Text Solution
|
- Which of the following compounds will react with sodium hydroxide solu...
Text Solution
|
- Phenol is less acidic than
Text Solution
|
- Which of the following is most acidic?
Text Solution
|
- Make the correct order of decreasing acid strength of the following co...
Text Solution
|
- Mark the correct increasing order of reactivity of the following compo...
Text Solution
|
- Arrange the following compounds in increasing order of boiling point :...
Text Solution
|