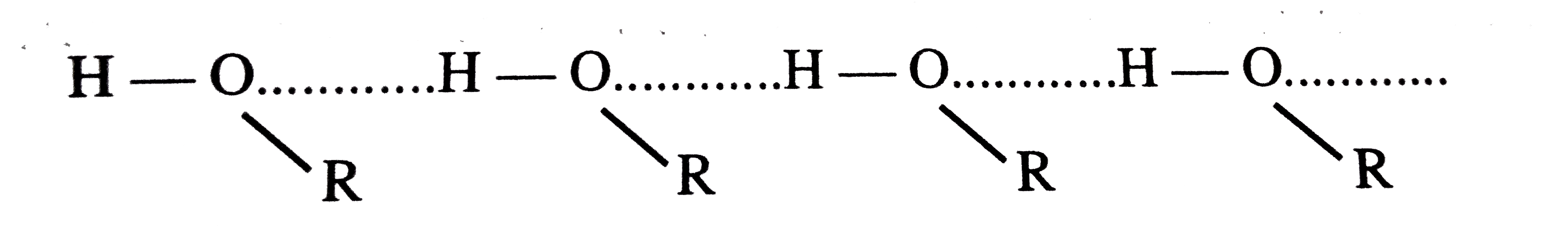Text Solution
Verified by Experts
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise ADDITIONAL QUESTIONS (SHORT ANSWER QUESTIONS)|81 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise ADDITIONAL QUESTIONS (LONG ANSWER QUESTIONS)|6 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise NCERT EXEMPLAR PROBLEMS WITH ANSWERS, HINTS AND SOLUTION (LONG ANSWER QUESTIONS)|4 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALCOHOLS, PHENOLS AND ETHERS-ADDITIONAL QUESTIONS (VERY SHORT ANSWER QUESTIONS)
- Arrange the four isomeric butyl alcohols in order of decreasing boilin...
Text Solution
|
- Arrange alkaline earth metal chlorides in order of increasing solubili...
Text Solution
|
- Why do alcohols have higher boiling points than haloalkanes of the sam...
Text Solution
|
- Anhydrous CaCl(2) is not recommended as a drying agent for alcohols an...
Text Solution
|
- What is the order of acidic character of primary, secondary and tertia...
Text Solution
|
- One mole of an organic compound (A) having M.F. C(2)H(6)O reacts with ...
Text Solution
|
- Why is sulphuric acid not used during the reaction of alcohols with KI...
Text Solution
|
- What is the order of reactivity of HCl, HBr and HI with alcohols?
Text Solution
|
- How will you convert ethanol into ethene?
Text Solution
|
- Explain the relative ease of dehydration of alcohols as : tertiary gt ...
Text Solution
|
- Name a reagent which converts primary alcohols exclusively to correspo...
Text Solution
|
- Name the main product obtained when vapour of tert-butyl alcohol are p...
Text Solution
|
- Vapours of an alcohol 'C' on passing over heated copper produce compou...
Text Solution
|
- name one reagent which is used for the distinction of primary, seconda...
Text Solution
|
- Arrange the following alcohols in order of increasing reactivity towar...
Text Solution
|
- Which of the following alcohols give iodoform test?
Text Solution
|
- How will you distinguish between 1-phenylethanol and 2-pheylethanol ?
Text Solution
|
- What is rectified spirit?
Text Solution
|
- Absolute alcohol is
Text Solution
|
- Power alcohol is :
Text Solution
|