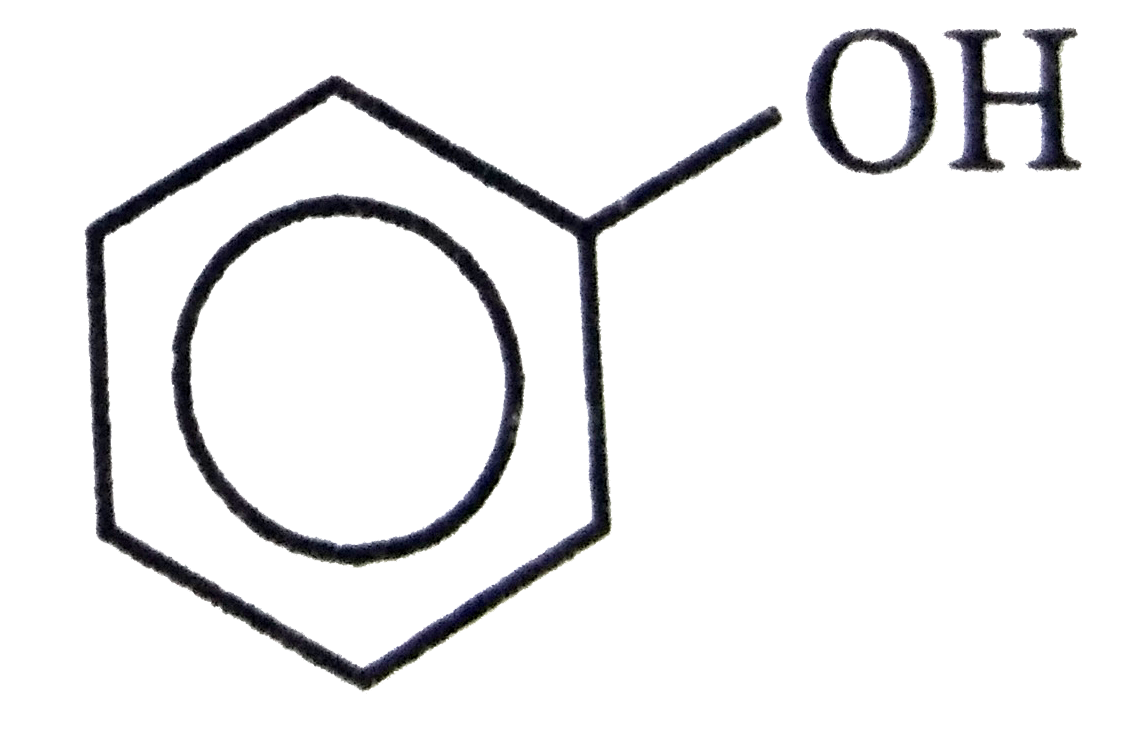
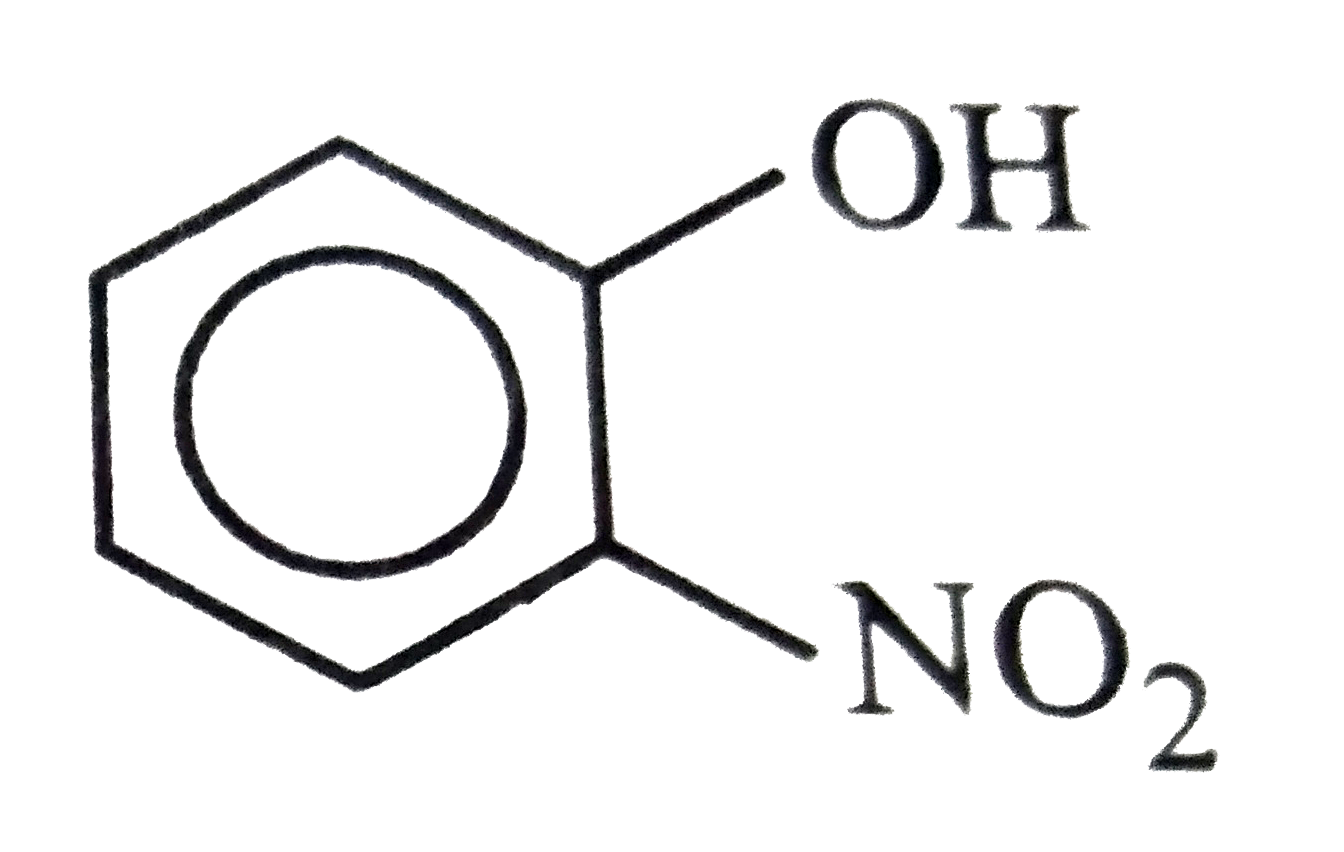
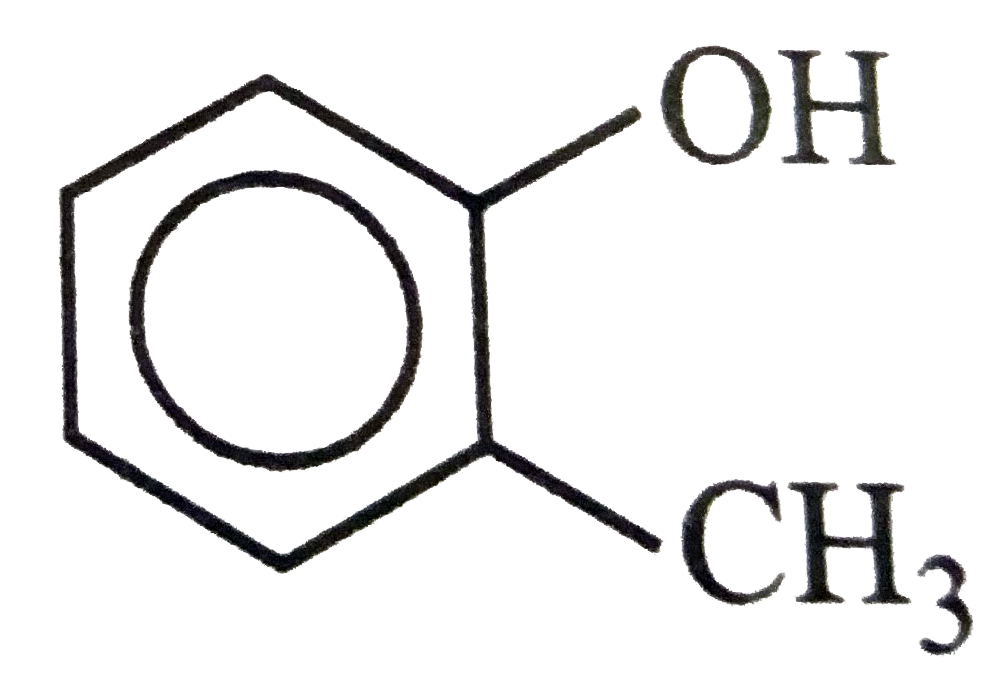
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
ALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER-II)|12 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MULTIPLE CHOICE QUESTIONS -III) Comprehension|8 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise VALUE BASED QUESTIONS WITH ANSWERS|4 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATION|29 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALCOHOLS, PHENOLS AND ETHERS-COMPETITION FOCUS JEE (MAIN AND ADVANCED)/MEDICAL ENTRANCES SPECIAL (MULTIPLE CHOICE QUESTIONS WITH ONE CORRECT ANSWER-I)
- On commercial scale, phenol is obtained from chlorobenzene by raschig'...
Text Solution
|
- The conversion of m-nitrophenol to resorcinol inivolves respectively:
Text Solution
|
- Which of the following compounds is most acidic?
Text Solution
|
- The correct order of decreasing acidity of nitrophenols will be
Text Solution
|
- The correct order of acid strength of the following substituted phenol...
Text Solution
|
- Arrange the following compounds is order of decreasing acidity.
Text Solution
|
- among the following four compounds (i) phenol (ii) methylphenol (i...
Text Solution
|
- Arrange the following compounds in increasing order of their acidic st...
Text Solution
|
- Strongest acid amon the following is
Text Solution
|
- Given are cyclohexanol (I), acetic acid (II),2,4,6-trinitrophenol (III...
Text Solution
|
- Which of the following will not be soluble in sodium bicarbonate?
Text Solution
|
- The product A will be
Text Solution
|
- Ortho-nitrophenol is less soluble in water than p- and m-nitrophenols ...
Text Solution
|
- The stability towards dehydration of the following compound decr...
Text Solution
|
- Which one is the most reactive towards electrophilic reagent?
Text Solution
|
- When phenol is treated with D(2)SO(4)//D(2)O, some of the hydrogens ge...
Text Solution
|
- The structure of the compound that gives a tribromo derivative on trea...
Text Solution
|
- The reaction of will HBr gives
Text Solution
|
- Phenol, when it first reacts with concentrated sulphuric acid and then...
Text Solution
|
- The major product obtained on interaction of phenol with sodium hydrox...
Text Solution
|