Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT SOLVED QUESTIONS)|5 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS)|8 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise TEST YOUR GRIP (II. FILL IN THE BLANKS)|53 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -CONCEPTUAL QUESTIONS
- Although aldehydes are easily oxidisable, propanal can conveniently be...
Text Solution
|
- Explain why dialkylcadmium is considered superior to Grignard reagent...
Text Solution
|
- Although and have a double bond, they exhibit different type of addi...
Text Solution
|
- Explain why aldehydes are more reactive than ketones.
Text Solution
|
- Benzaldehyde reduces Tollens' reagent but not the Fehling's or the Ben...
Text Solution
|
- Dipole momentts of aldehydes and ketones are higher than those of alco...
Text Solution
|
- Explain, why o-hydroxybenzaldehyde is a liquid at room temperature whi...
Text Solution
|
- Identity A,B and C in the following reaction HC-=CH underset(HgSO(4)...
Text Solution
|
- Suggest a suitable oxidising agent for the given conversion. (CH(3))...
Text Solution
|
- Write the structures (A) to (C) for the following reactions: .
Text Solution
|
- An organic compound (A) having moleclar formula C(2)H(6)O on oxidation...
Text Solution
|
- Show the arrowhead steps for the preparation of acetic acid by using ...
Text Solution
|
- Fluorine is more electronegative than chlorine but p-fluorobenzoic aci...
Text Solution
|
- p-Nitrobenzoic acid has higher K(a) value than benzoic acid. Give reas...
Text Solution
|
- Carboxylic acids donot give the characteristic reactions of carbonyl g...
Text Solution
|
- Predict the product of the following reaction
Text Solution
|
- Identify A to E in the following reaction
Text Solution
|
- Identify A to E in the following series of reactions:
Text Solution
|
- Write the structure of A,B,C,D and E in the following reactions:
Text Solution
|
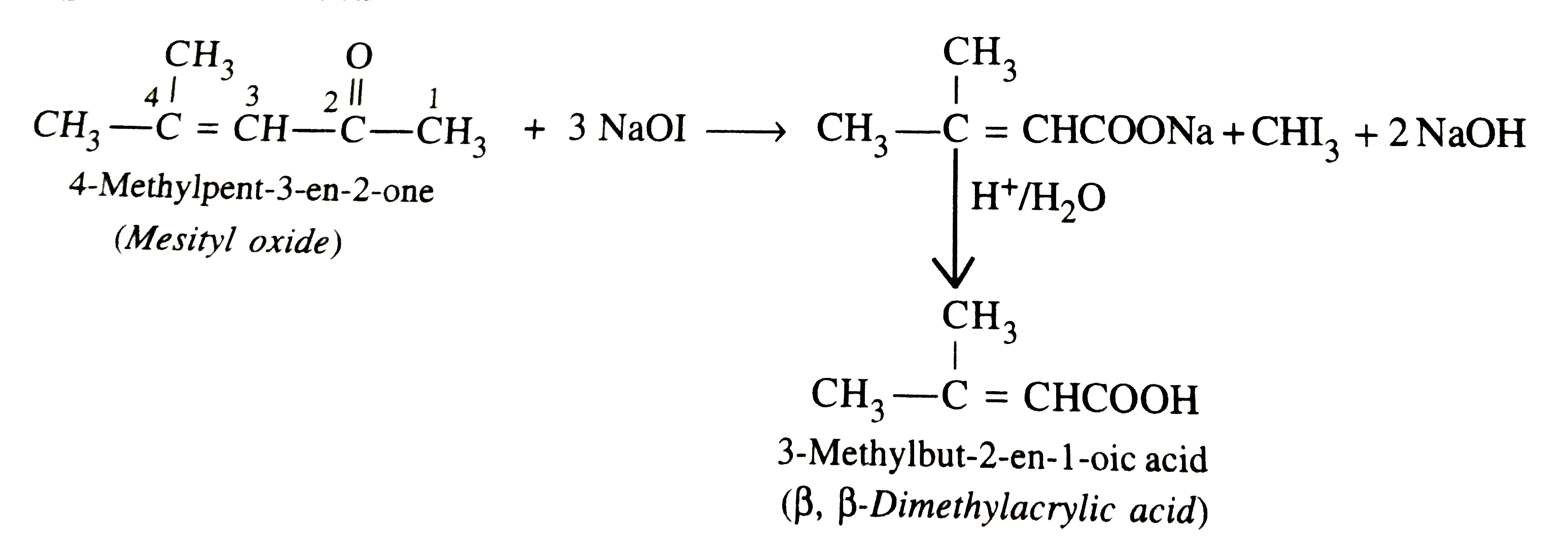 .
.