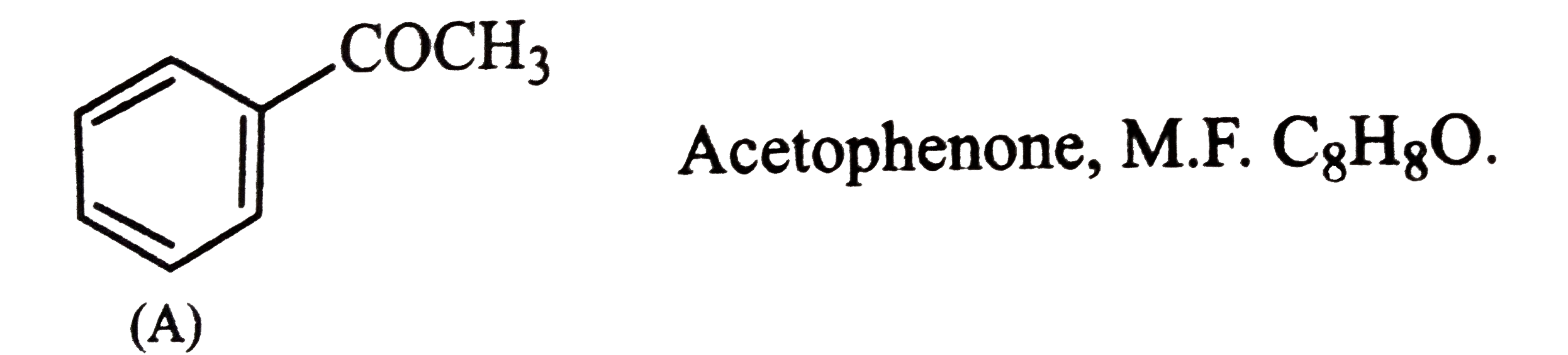Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT UNSOLVED QUESTIONS)|8 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise NCERT EXERCISES|20 VideosALDEHYDES, KETONES AND CARBOXYLIC ACIDS
PRADEEP|Exercise CONCEPTUAL QUESTIONS|19 VideosALCOHOLS, PHENOLS AND ETHERS
PRADEEP|Exercise IMPORTANT QUESTIONS FOR BOARD EXAMINATIONS.|29 VideosAPPENDIX
PRADEEP|Exercise MODEL TEST PAPER <br> (Section C )|15 Videos
Similar Questions
Explore conceptually related problems
PRADEEP-ALDEHYDES, KETONES AND CARBOXYLIC ACIDS -NCERT QUESTIONS AND EXERCISES WITH ANSWERS (NCERT INTEXT SOLVED QUESTIONS)
- Give names of the reagents to bring about the following transformation...
Text Solution
|
- Arrange the following compounds in the increasing order of their boili...
Text Solution
|
- Would you expect benzaldehyde to be more reactive or less reactive in ...
Text Solution
|
- An organic compound (A) with molecular formula C8H8 O forms an o...
Text Solution
|
- Write chemical reactions to affect the following transformations: (i...
Text Solution
|